Christopher H. Contag and Ahmed A. Zarea from Michigan State University’s Institute for Quantitative Health Science and Engineering explore therapeutic approaches to neurodegenerative diseases using a novel strategy based on engineered endosymbionts systems that could revolutionize patient care
Neurodegenerative diseases are characterized by the progressive loss of neurons and their function; in the case of Parkinson’s disease (PD), dopaminergic neurons are lost. This leads to significant deficits in motor control and cognitive function. Neuronal degeneration in PD primarily affects neurons in the substantia nigra, a brain region crucial for regulating movement. Current therapies, including the drug Levodopa and deep brain stimulation, primarily alleviate symptoms rather than address the underlying causes of neuronal death. (1)
The discovery of induced pluripotent stem cells (iPSCs) in 2006 has advanced the field of regenerative medicine and is beginning to offer hope for the treatment of neurodegenerative diseases. (2) Stem cell therapies, which involve transplanting neurons derived from stem cells into damaged brain areas, have the potential to restore lost neuronal function. However, these approaches are limited by immune rejection, poor survival of transplanted cells, and the inability to integrate new neurons into existing neural networks. (3)
Cellular reprogramming technologies, which convert existing cells within a patient’s body into functional neurons, are being investigated in situ. In these approaches, astrocytes or neural progenitor cells are reprogrammed directly, bypassing the need for transplantation and reducing the risks of immune rejection. Although promising, in vivo reprogramming faces challenges related to precise cellular targeting, effective control, durable effects, and safety.
Engineered bacteria for cell reprogramming
Methods for gene delivery for cell reprogramming in vivo often utilize viral vectors. These vectors pose risks such as insertional mutagenesis and irreversible transformation, leading to cancer. An emerging alternative gene delivery tool utilizes engineered bacteria, which can deliver reprogramming factors without integrating into the host genome, thereby avoiding the risk of insertional mutagenesis and providing additional means of controlling function for improved safety.
Certain pathogens have been shown to naturally induce reprogramming of host cells, supporting this notion. For example, studies by Hess and Rambukkana (2015) demonstrated that Mycobacterium leprae can reprogram Schwann cells into a stem cell-like state, facilitating bacterial spread while revealing potential regenerative pathways. (4) Similarly, Mycobacterium leprae has been shown to reprogram adult Schwann cells in vivo, promoting their proliferation and migration. (5) Although this process leads to increased infection, it parallels potential therapeutic strategies and suggests that bacteria could induce regenerative states without needing external stem cell transplants. In support of engineered bacteria being used for directed reprogramming, Hess (2022) showcased engineered bacteria that induce partial reprogramming in the liver, promoting growth without causing fibrosis or tumorigenesis. These engineered bacteria facilitated liver cell regeneration while avoiding risks associated with complete reprogramming. (6)
These studies emphasize the potential of bacteria as innovative tools for in vivo cellular engineering and cell reprogramming, offering safer and more controlled methods for tissue regeneration. Harnessing bacterial mechanisms for inducing stem cell-like states is a new avenue for treating degenerative diseases and repairing tissue damage. Extending this concept, we have designed bacteria to reside in the host cytoplasm as engineered endosymbionts, which deliver transcription factors to the host nucleus to guide cellular function. (7,8) It is possible to use engineered bacteria for precise and temporary expression of reprogramming factors, minimizing off-target effects and enhancing safety.
Engineered endosymbionts for guided tissue regeneration
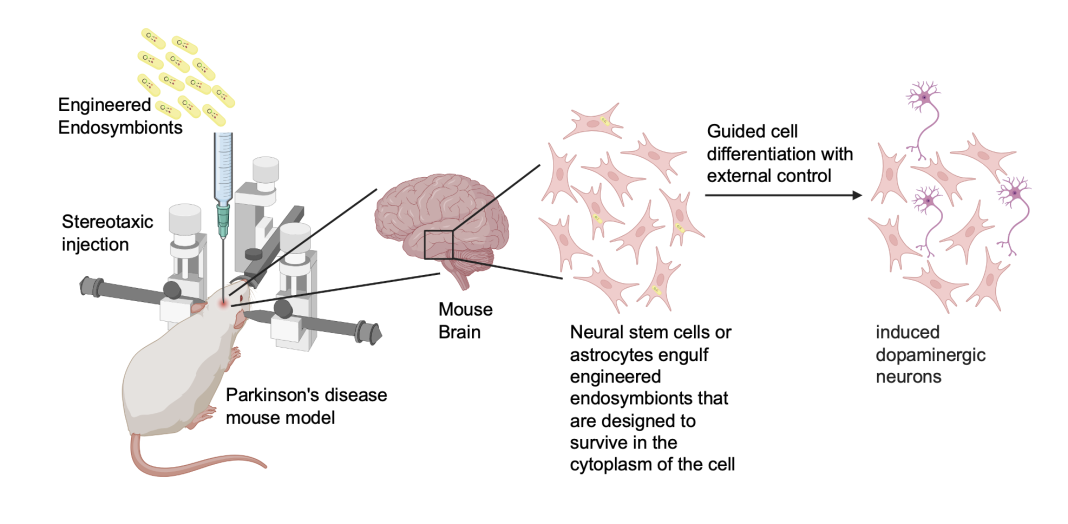
In the context of Parkinson’s disease, a novel strategy is emerging that focuses on reprogramming resident astrocytes into dopaminergic neurons. (9) Astrocytes, a type of glial cell in the brain, are abundant and play a crucial role in supporting neuronal function. Given their accessibility, potential for transformation, and propensity to take up bacteria through endocytosis, they are ideal candidates for reprogramming via engineered endosymbionts.
Engineered bacteria have been developed to deliver proteins to the extracellular matrix, facilitating cell uptake to induce differentiation and reprogramming. (10) Further, engineered bacteria designed to persist in the cytoplasm of host cells, where they express and secrete proteins with nuclear localization signals, can control genetic pathways for cellular reprogramming. (8) As such, engineered endosymbionts have the ability to control the timing and quantity of factor delivery through inducible gene regulation. This precision allows for targeted and transient reprogramming, essential for minimizing potential side effects and ensuring the procedure’s safety. By delivering specific transcription factors that promote dopaminergic neuron production— such as ASCL1, LMX1A, and NURR1 (11) —endosymbionts can facilitate the generation of functional neurons crucial for treating Parkinson’s disease.
However, challenges remain in the form of potential immune responses against engineered endosymbiotic systems (EES). The host’s immune system will recognize these bacteria as foreign invaders, which could compromise their delivery; however, once they reside within host cells, they may be protected. EES have been engineered that have improved intracellular survival through the expression of listerialysin O (LLO), a protein derived from Listeria monocytogenes. (12) This protein enables bacteria to escape from the phagolysosome into the cytoplasm, enabling survival and secretion of transcription factors necessary for cellular reprogramming. This strategy aims to enhance the intracellular persistence of engineered bacteria, and further modifications may lead to a more symbiotic relationship, thereby improving the duration and overall effectiveness of the treatment.
Establishing a balance between EES and host cell survival to ensure the bacteria persist long enough to deliver the requisite proteins for effective cellular reprogramming will advance this therapeutic approach. Additionally, improved control of bacterial gene expression using signals from outside the body, (13) such as magnetothermal energy, and increasing the complexity of controlled pathways will improve cellular reprogramming.
Magneto-endosymbionts
Fine-tuning the control of gene expression in the engineered endosymbionts will lead to greater precision and improved safety. Both chemical and thermal control have been demonstrated, and each can be utilized to remotely modulate gene expression, facilitating the targeted delivery of therapeutic proteins. (8,13) Magneto-endosymbionts are engineered bacteria with magnetic particles that respond to alternating magnetic fields with changes in gene expression. (13) This innovative approach has the potential to overcome many of the current limitations. Engineered endosymbionts offer revolutionary strategy for tissue regeneration in the context of neurodegenerative diseases.
By harnessing the unique properties of bacteria to deliver transcription factors directly to nuclei of astrocytes is a powerful stregey for guiding regenerative medicine.As we continue to explore the intricacies of these systems, the potential for developing safe and effective treatments for Parkinson’s disease and other neurodegenerative conditions becomes increasingly within reach.