Renea Creech and Kim Wilson outline the challenges of Chronic Kidney Disease in pets, the irreversible loss of kidney function, and how nutrition can help
Approximately one in three cats and one in ten dogs are likely to develop kidney disease in their lifetime. (1,2) Increased incidence of diagnosis occurs in older pets, particularly in cats. (3,4) A longevity study showed renal disorders were a major cause of death in cats over five years (5), and upwards of 80% aged over 15 years are affected by Chronic Kidney Disease (CKD) or other renal disorders. (3,4)
Clinical signs include vomiting, decreased appetite, increased water intake, and total and lean body mass loss. Veterinarians often analyse urine and blood samples to determine biomarker levels such as serum creatinine and symmetric dimethylarginine (SDMA). The International Renal Interest Society provides veterinarians with guidance for diagnosing and managing kidney disease based on staging, with stage 1 representing early CKD and stage 4 severe CKD. (6)
Diagnosing CKD in pets is challenging partly because kidneys adapt to the structural and functional loss of nephrons and renal tubules to maintain glomerular filtration rate and eliminate waste such as urea, indoles, phenols, and other nitrogenous waste. (7) Significant kidney damage often occurs before clinical signs appear, (7) and lifespan is ultimately shortened.
Nutritional management is recommended for pets diagnosed with CKD to slow progression and ensure adequate nutrient intake. Prescribed by a veterinarian, these foods are formulated to have controlled protein, phosphorus, and sodium to reduce the workload of the kidneys and added omega-3 fatty acids and antioxidants to fight inflammation. These foods can be different for early stage (1 & 2) and late stage (3 & 4) by levels of nutrients provided.
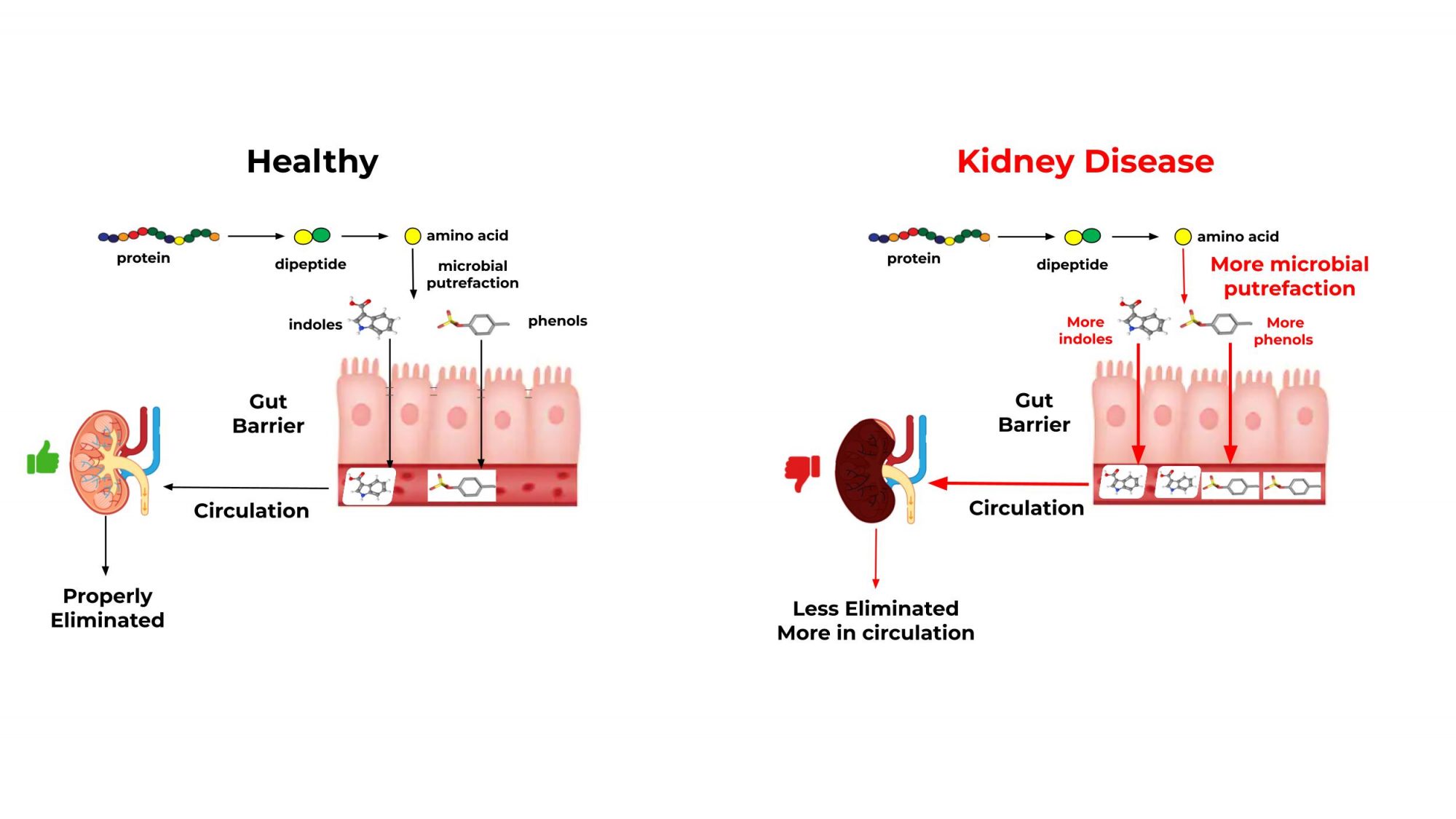
The gut microbiome-renal axis influence on CKD
Like the gut-brain axis, crosstalk between the gut and kidneys impacts CKD. (7) People and animals with CKD have less gut microbiome diversity. (9-11) The gastrointestinal microbiome shows functional differences between healthy and CKD pets (see Figure 1).
In these pets, there is a decrease in protein digestion, causing microbiome activity to shift towards putrefaction, which is the fermentation of undigested protein in the gut. This causes an increase in the production of end-products such as indoxyl-3 sulfate and p-cresyl sulfate. These circulating end products normally enter the kidneys as uremic solutes; however, the build-up is toxic (uremic toxins). (12)
A healthy kidney with abundant nephrons (7) and functional renal tubules can eliminate solutes. However, pets with CKD have a reduced ability to eliminate uremic solutes, which become toxins.
The prolonged inflammation in the kidneys and the gut caused by these putrefactive end-products can disrupt the intestinal barrier and the kidneys’ ability to eliminate uremic toxins. Sustained inflammation leads to further damage of the kidneys and increases gut permeability (12,13), making it harder for pets with CKD to absorb nutrients, leading to weight loss and lean body mass. (14) The progression of CKD is thought to result from this self- perpetuating cycle, which can be combated by nutritional interventions, specifically by feeding the gut microbiome.
Feeding the microbiome to slow the progression of CKD in pets
Feeding the microbiome impacts both the pets’ nutrient digestion and the microbiome’s end-products. (15) Fiber is an excellent substrate for the microbiome as multiple meta-analyses have shown fiber intake reduces uremic toxins (p-cresyl sulfate and indoxyl sulfate) in people with CKD. (16,17) Selecting appropriate fibers is important to enhance specific microbiome functions. (18)
For pets with CKD, the goal is to reduce the microbiome’s putrefaction end- products while promoting beneficial end-product production and limiting the inflammatory response. Fibers in small quantities are required to maintain the strict nutritional formulation for pets with CKD.
Novel nutritional approaches to manage CKD
Fiber
Several fermentable types of fiber have shown promising results in kidney disease. Fructooligosaccharides (FOS, short linear fructose oligomers) and oat beta-glucan (OBG, larger, nonlinear polysaccharides) reach the colon where putrefaction occurs and provide the microbiome substrates to promote beneficial activity and reduce putrefactive end-products.
Providing FOS or OBG has reduced uremic toxins p-cresyl sulfate and trimethylamine N-oxide (TMAO) in people with CKD, respectively. (19,20) Mixtures of fibers provided by ingredients such as apple pomace additionally contain antioxidants in the form of polyphenols. (20) Apple pomace can increase intestinal SCFA and lower ammonia production in kidney-injured rats. (21)
A recent study evaluated feeding cats with CKD foods containing OBG and either apple pomace or FOS. (22) Both fiber sources increased bacterial abundance of SCFA-producing bacteria relative to cats not provided with treatment fiber. More importantly, the microbiome activity dramatically changed when comparing fiber sources. Food with OBG-FOS reduced circulating creatinine and several uremic toxins in cats with CKD compared to OBG-apple pomace. (23) Therefore, a well-selected and more readily fermented fiber source may be better for pets with CKD.
To support an animal with CKD, a new approach is needed to nourish the microbiome properly and support pets’ kidneys and gut at the cellular level.
Osmolytes
Osmolytes protect cell structure from damage and death by facilitating water uptake and retention, relieving cells from the additional energy required to maintain homeostasis. Betaine is an osmolyte that is efficiently utilised in the kidneys and gut. Betaine has specific receptors (BGT-1) in the kidneys and gut for uptake.
Unlike other osmolytes, betaine spares choline and methionine nutrients, reducing homocysteine reserves, which is considered a uremic toxin in pets with CKD. Circulating levels of betaine naturally decrease as CKD progresses (25); dietary betaine supports kidney cells and improves intestinal integrity (26) needed in pets with CKD.
Recent publications on cats with CKD tested changes in gut microbiome activity and body composition when fed the fibers FOS, OBG and the osmolyte betaine in a food designed for pets with kidney disease. (27) The betaine-FOS-OBG addition increased total body mass in cats with CKD when compared to the control kidney food without betaine-FOS-OBG addition.
A more extensive CKD cat study saw improved total and lean body mass using the same betaine-FOS-OBG. (28) A similar study in CKD dogs showed total and lean body mass maintenance after over two months on the same betaine-FOS-OBG. (29)
Across these studies, reductions of several uremic toxins were found, including 3-indoxyl sulfate, several carnitine derivatives, a source of TMAO, and homocysteine while increasing omega-3 fatty acids DHA and DPA. (27-29)
Traditional nutritional solutions for pets with CKD include controlling protein, phosphorus, and sodium to reduce kidney stress. Nutritional approaches that affect the gut microbiome-renal axis can have profound positive effects on pets with CKD. Foods with proper fibers and osmolytes will support the gut- microbiome renal axis as an additional approach to increase the quality of life for pets with CKD.
References
- Lulich JP, Osborne CA, O’Brien TD, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet. 1992;14(2):127–153
- https://www.msdvetmanual.com/mvm/urinary-system/%20noninfectious-diseases-of-the-urinary-system-in-small-animals/renal-%20dysfunction-in-small-animals
- https://journals.sagepub.com/doi/10.1177/1098612X13511446
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7799420/
- https://journals.sagepub.com/doi/10.1177/1098612X14536176?url_ver=Z39.88- 2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- http://www.iris-kidney.com/guidelines/staging.html
- https://bvajournals.onlinelibrary.wiley.com/doi/abs/10.1136/inp.i4914
- https://www.nature.com/articles/s41581-018-0018-2
- https://onlinelibrary.wiley.com/doi/full/10.1111/jvim.15389
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3968507/
- https://pubmed.ncbi.nlm.nih.gov/22992469/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5086447/
- https://journals.lww.com/jasn/Fulltext/2017/01000/Intestinal_Dysbiosis,_Barrier_Dysfunction,_and.13.aspx
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6071212/
- https://www.openaccessgovernment.org/article/fibers-microbiome-postbiotics-pet-health/150249/
- https://www.sciencedirect.com/science/article/pii/S0261561418324531?via%3Dihub
- https://www.sciencedirect.com/science/article/abs/pii/S1051227620302910?via%3Dihub
- https://doi.org/10.3389/fmicb.2020.01266
- https://academic.oup.com/ndt/article/34/11/1876/5042969
- https://www.mdpi.com/2227-9717/8/3/319
- https://pubs.acs.org/doi/pdf/10.1021/jf201950y
- https://www.sciencedirect.com/science/article/abs/pii/S1051227619302705?via%3Dihub
- http://dx.doi.org/10.3390/metabo10070281
- https://www.mdpi.com/2076-2615/12/6/768
- https://link.springer.com/article/10.1007/s11255-020-02632-6
- https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-020-02298-3#Bib1
- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0268624
- https://www.imrpress.com/journal/FBE/15/2/10.31083/j.fbe1502008/htm
- https://www.mdpi.com/2218-1989/10/9/370

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


