Variability in cochlear implant performance remains a significant challenge for clinicians and patients. Contributors from Washington University School of Medicine investigate how surgical techniques and intraoperative adjustments can be refined to further optimize cochlear implant outcomes
Building on our previous findings – where we demonstrated that both cochlear health (assessed via electrocochleography) and cognitive function are key determinants of CI performance (read here and here) – this article shifts its focus to the implantation of the device itself covering how surgical techniques and intraoperative adjustments can be modified to further optimize CI outcomes.
Impact of angular insertion depth and wrapping factor on CI performance
Modern cochlear implant systems offer a wide range of electrode designs that vary in length, rigidity, electrode count, spacing, stimulation strategy, and intracochlear positioning. For example, lateral wall electrodes – positioned farther from the central neural structures –are generally associated with better hearing preservation, whereas perimodiolar electrodes are engineered to contour closely to the modiolus, potentially reducing current requirements and enhancing performance, though with a theoretical risk of increased intracochlear trauma. Optimizing outcomes has been related to both sufficient cochlear coverage (i.e., adequate angular insertion depth) and an optimal wrapping factor (i.e., perimodiolar proximity). Moreover, the preservation of residual hearing adds significantly to performance without the need for deep cochlear insertion. Unfortunately, none of the modern electrode arrays can reliably accomplish deep angular insertion depth, perimodiolar position, and hearing preservation.
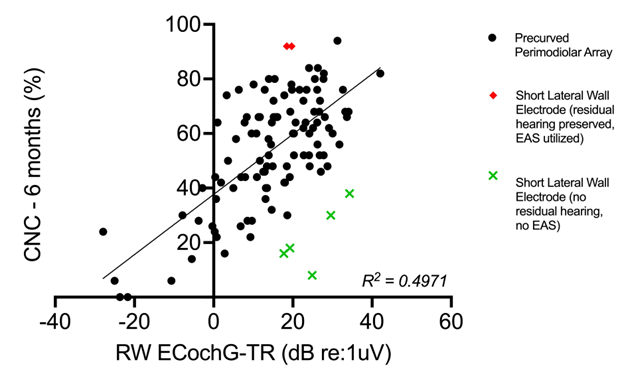
By integrating these surgical variables (i.e., electrode position) while controlling for cochlear health, we better understand their collective impact on CI performance. In our work, subjects with shallower lateral wall electrode array insertions who retained residual hearing demonstrated superior CI performance (1-3) (Figure 1). Conversely, when hearing preservation was not achieved, the same shallow insertions correlated with below-average outcomes when compared to perimodiolar arrays. Unfortunately, predicting which patients will preserve hearing remains challenging. Ideally, a shallow insertion would be performed in hearing preservation cases, with the option to advance the electrode further if needed. In this context, technologies such as electrocochleography (ECochG) hold promise for providing feedback on intraoperative cochlear trauma and potentially enhancing hearing preservation, ultimately improving overall CI performance.
Using electrocochleography to minimize intracochlear trauma and optimize electrode placement for improved hearing preservation and CI performance
We have explored intraoperative ECochG as a tool to monitor and optimize hearing preservation for both lateral wall (4,5) and perimodiolar electrodes. (6,7) With lateral wall electrodes, once the stimulus parameters are clearly established, the approach is relatively straightforward. Traditionally, nearly every study evaluating ECochG during electrode insertion employed a 500Hz acoustic stimulus and recorded responses from the most apical electrode to detect amplitude drops that signify intracochlear trauma or basilar membrane impact. Our recent findings suggest that for electrodes inserted beyond 350 degrees, a 250Hz stimulus is more appropriate. (6) This adjustment is necessary because it remains unclear whether a drop in intensity after 350 degrees reflects crossing the tonotopic place for 500Hz or contact with the basilar membrane. Understanding and incorporating this parameter, alongside further understanding of ECochG-based responses and phases, will be essential for effective ECochG-guided hearing preservation protocols.
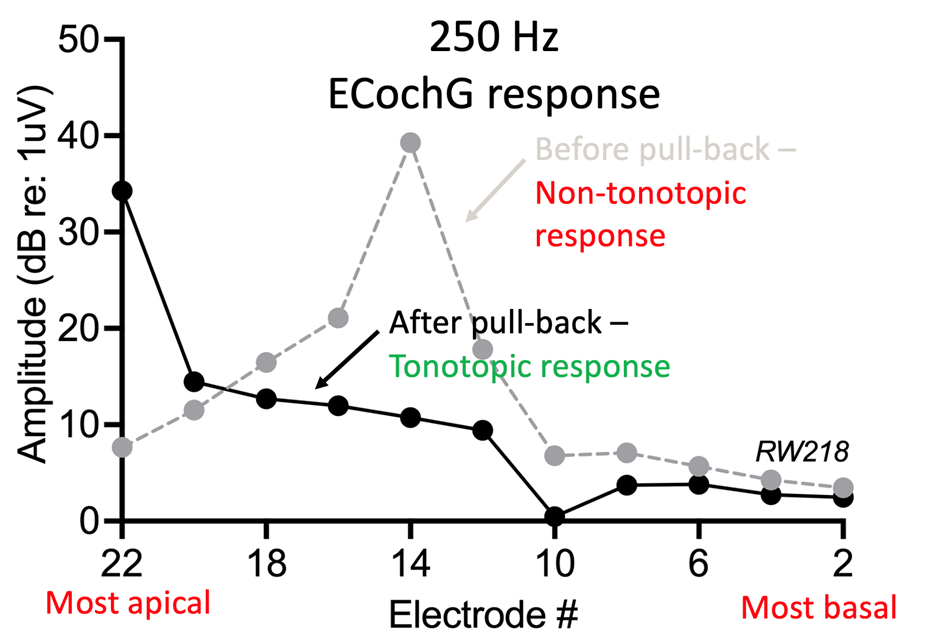
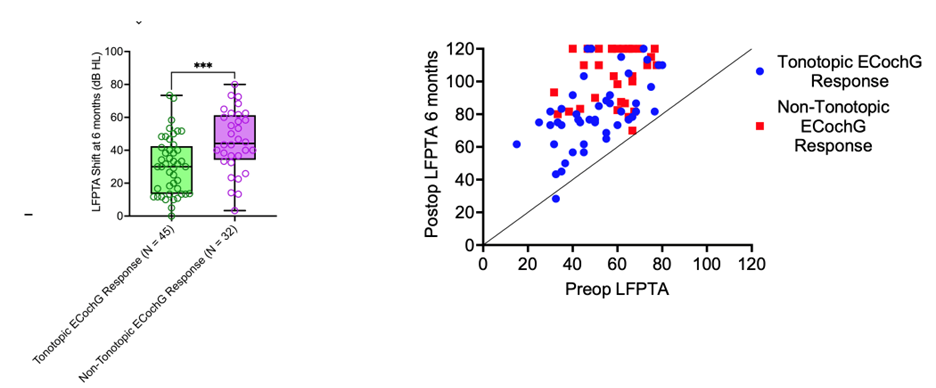
For lateral wall electrodes, a decline in the 250Hz response should prompt a pause in insertion with appropriate feedback adjustments. In contrast, perimodiolar electrodes have traditionally been considered less amenable to hearing preservation due to challenges in pausing insertion – given the minimal tactile feedback and the insertion via a sheath. To address this, we developed a post-insertion modification approach for perimodiolar electrodes. (7) This technique involves intentionally over-inserting the electrode, then using ECochG to assess whether the response is tonotopically distributed (with 250Hz registering at the most apical electrode, 500Hz slightly basal, and higher frequencies shifting further basally, as expected from the cochlea’s tonotopic organization; see Figure 2). If the response is non-tonotopic, the electrode is intentionally pulled back to minimize contact with the basilar membrane, improve its proximity to the modiolus, and ultimately optimize the tonotopic ECochG response. Our data demonstrate that this method enhances perimodiolar proximity, improves hearing preservation (Figure 3), and leads to better performance in noise.
Future directions: advancing CI performance stratification – integrating surgical factors, cochlear health, and cognitive function into predictive modeling
The variability in CI performance remains a significant challenge for clinicians and patients alike, often resulting in a reactive rather than proactive approach to managing suboptimal outcomes. Throughout this series, we have highlighted key contributors to successful CI performance. Moving forward, it will be crucial to systematically integrate these clinical, surgical, and biological features into predictive models that enable more precise stratification of CI performance and improved patient outcomes.
First, cochlear health plays a fundamental role in CI success, with ECochG providing a robust and reliable biomarker for cochlea integrity and nerve function. Equally important is optimal brain function, as cognitive function significantly influences how the brain can adapt to effectively processing CI signals. This final section emphasizes how precise electrode placement tailored to individual anatomy and audiologic profiles is essential for maximizing performance. Incorporating these elements – cochlear health, cognitive function, device characteristics, and surgical technique – into predictive modeling enables a more comprehensive understanding of CI performance. Such personalized models will facilitate a more nuanced understanding of intervention impacts, enhance patient counseling, set realistic expectations, and identify individuals at risk for underperformance. Furthermore, they will support early detection of device-related challenges, guide tailored auditory rehabilitation strategies, and optimize clinical workflows. Ultimately, these integrative models will refine personalized CI interventions, improve long-term outcomes, and enable more accurate risk stratification for future clinical trials, ushering in a new era of precision medicine in cochlear implantation.
References
- Walia A, Shew MA, Lefler SM, et al. Factors Affecting Performance in Adults With Cochlear Implants: A Role for Cognition and Residual Cochlear Function. Otol Neurotol. Dec 1 2023;44(10):988-996. doi:https://doi.org/10.1097/mao.0000000000004015
- Walia A, Shew MA, Varghese J, et al. Improved Cochlear Implant Performance Estimation Using Tonotopic-Based Electrocochleography. JAMA Otolaryngol Head Neck Surg. Dec 1 2023;149(12):1120-1129. doi:https://doi.org/10.1001/jamaoto.2023.2988
- Walia A, Shew MA, Kallogjeri D, et al. Electrocochleography and cognition are important predictors of speech perception outcomes in noise for cochlear implant recipients. Sci Rep. Feb 23 2022;12(1):3083. doi:https://doi.org/10.1038/s41598-022-07175-7
- Walia A, Shew MA, Ettyreddy A, et al. Early Hearing Preservation Outcomes Following Cochlear Implantation With New Slim Lateral Wall Electrode Using Electrocochleography. Otol Neurotol. Apr 1, 2022;43(4):443-451. doi:https://doi.org/10.1097/mao.0000000000003475
- Walia A, Shew MA, Ortmann AJ, Buchman CA, Herzog JA. Hearing Preservation After Cochlear Reimplantation Using Electrocochleography: A Case Report. Laryngoscope. Oct 2021;131(10):2348-2351. doi:https://doi.org/10.1002/lary.29734
- Walia A, Shew MA, Lefler SM, et al. Is Characteristic Frequency Limiting Real-Time Electrocochleography During Cochlear Implantation? Front Neurosci. 2022;16:915302. doi:https://doi.org/10.3389/fnins.2022.915302
- Walia A, Shew MA, Lee DS, et al. Electrocochleography- Guided Pull-Back Technique of Perimodiolar Electrode for Improved Hearing Preservation. Otol Neurotol. Jan 22 2025;doi:https://doi.org/10.1097/mao.0000000000004407