JoAnne S Richards, PhD, Professor at Baylor College of Medicine, explores ovarian cancer research with a focus on ovarian function and dysfunction
The ovary is the female gonad responsible for supporting 1) follicular development, 2) ovulation of fertilizable oocytes/eggs and 3) luteinization to maintain viable offspring during pregnancy. Ovarian follicles produce estradiol that induces the surge of pituitary luteinizing hormone (LH); this initiates ovulation and the release of a fertilizable egg/oocyte. After ovulation, follicular cells (granulosa/theca) form progesterone producing corpora lutea that maintain pregnancy if a viable embryo implants (Figure1).
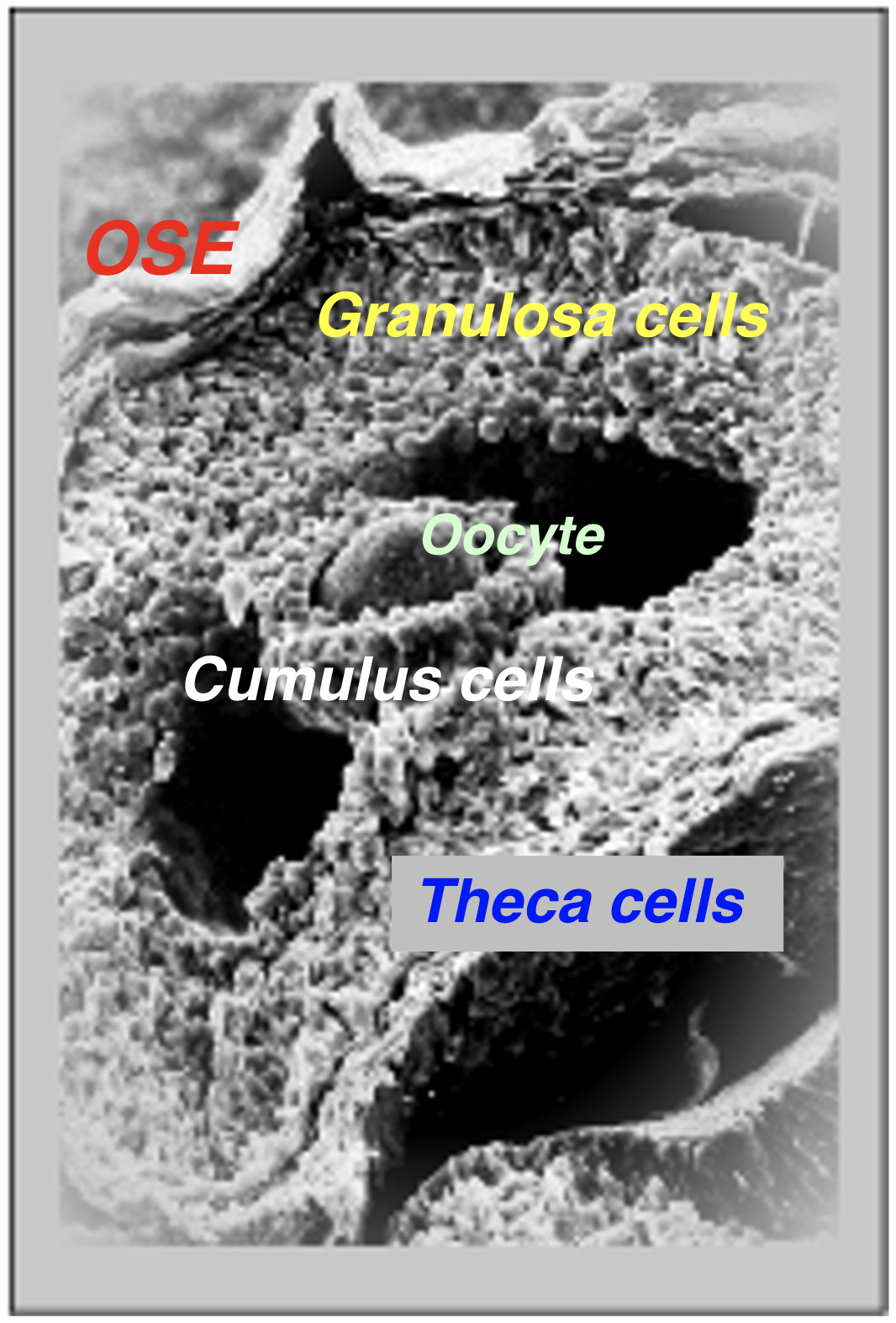
Ovarian cancer is largely a misnomer because the cells giving rise to malignant tissue are not derived from either follicular or luteal cells.
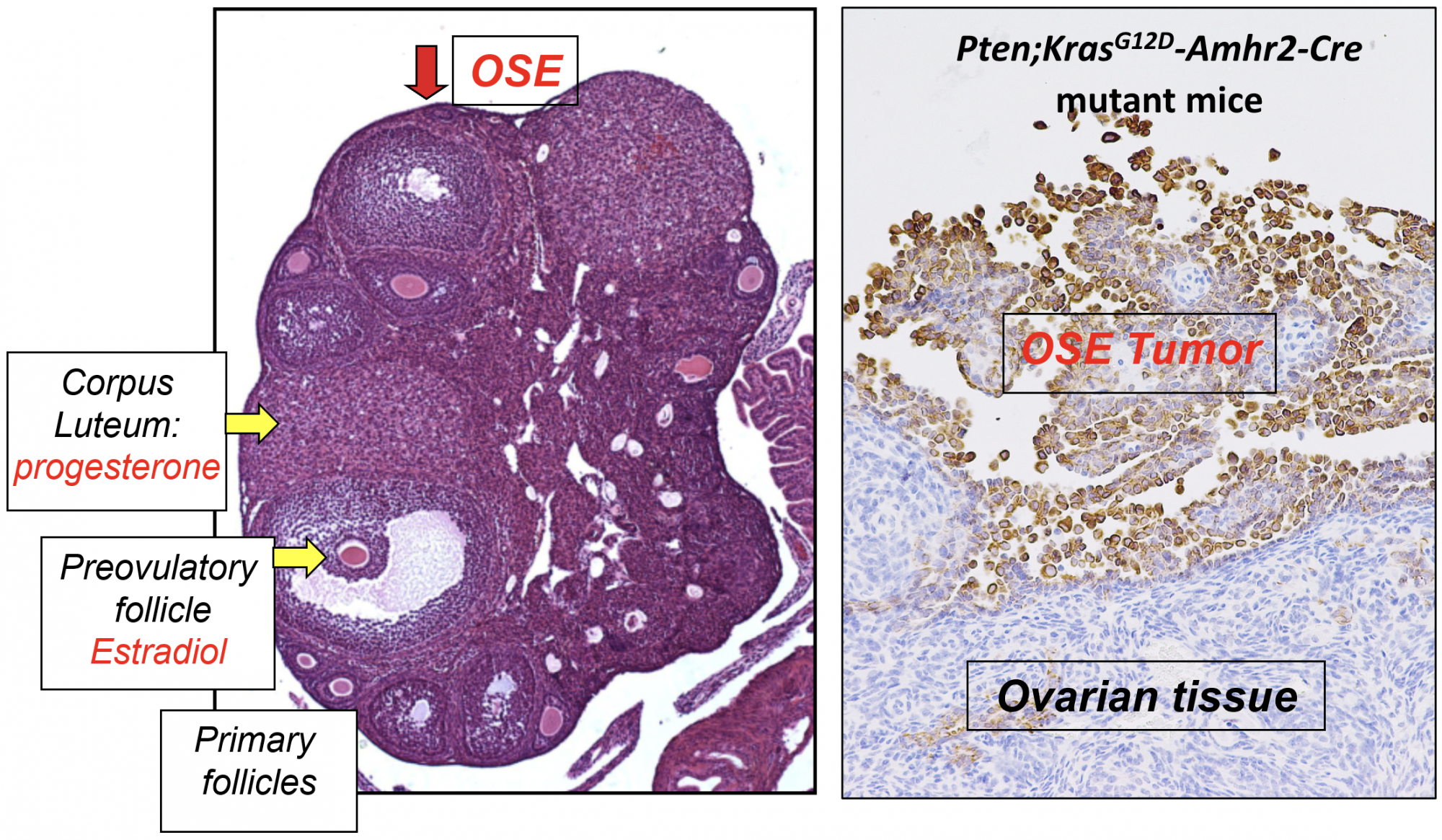
Instead, ovarian cancer cells evolve from ovarian surface epithelial cells (OSEs) that cover the surface of the ovary and fallopian tube and separate these tissues from the abdominal peritoneal cavity. Unlike other cancers that metastasize through the vascular system, ovarian cancer cells detach from the ovarian surface, disseminate and grow in the peritoneal cavity (Figure 2).
What are the causes of ovarian cancer? The answers to this question are complex. There is not just one, but many underlying factors. However, the most common cause that initiates cancer involves mutations in specific genes, such as tumor protein 53 (TP53), BRAC1/2, KRAS, PTEN, that lead to abnormal cell proliferation and evasion of cell death. Many of these genes are involved in DNA repair, essential for maintaining normal gene expression; others are involved in metabolic/ growth regulatory pathways.
Our studies in mice documented that mutations in p53, Kras and Pten in ovarian surface epithelial cells led to epithelial ovarian cancer and provided us with the first model of ovarian cancer that occurred early and was 100% penetrant (Figure 2). By depleting the tumor repressor protein 53 (Trp53; p53) gene in the Kras/Pten mutant mice, we discovered that wild type p53 was essential for tumor growth, whereas cells lacking p53 developed only small tumor lesions.
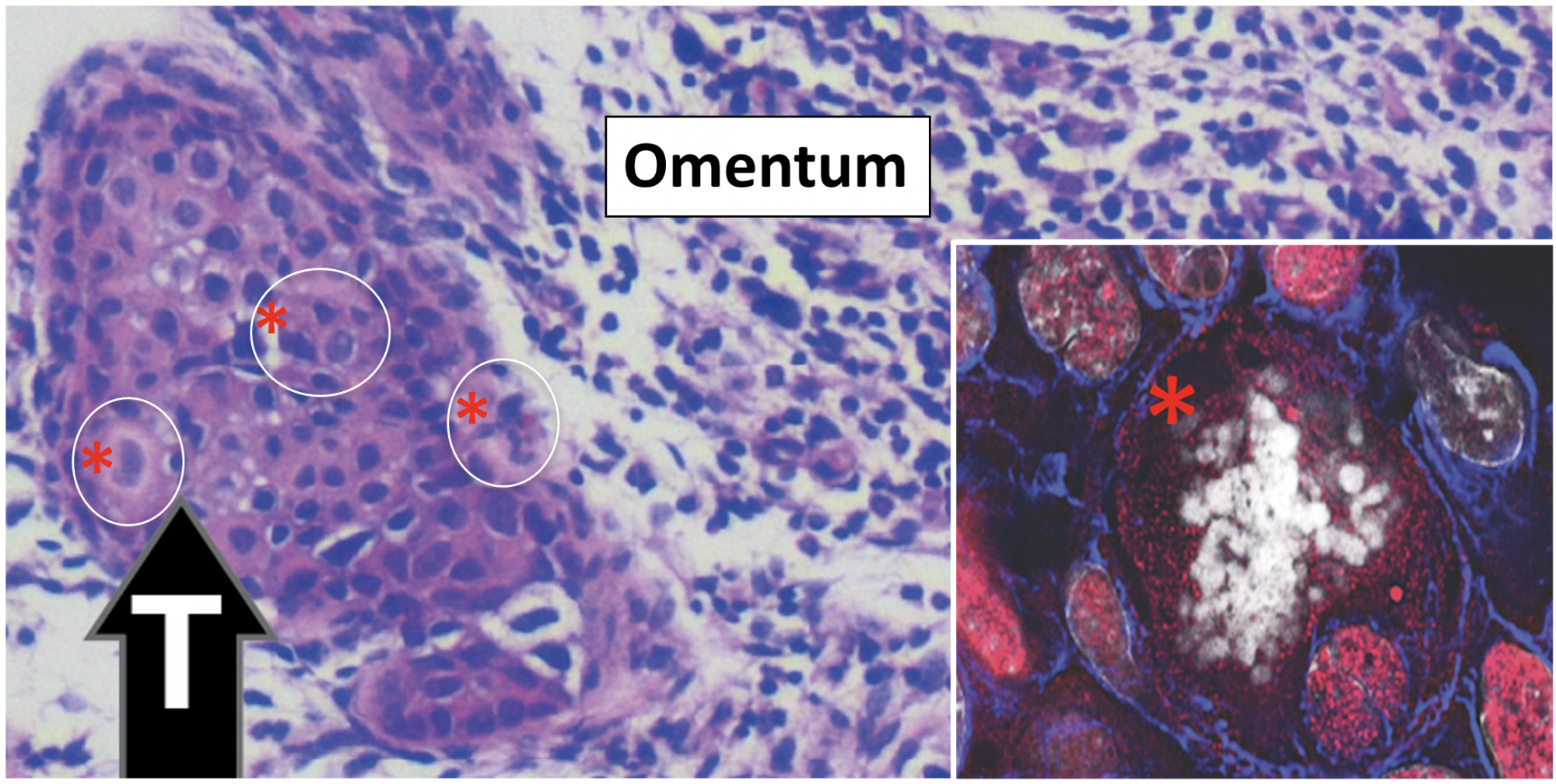
By contrast, p53 null epithelial cells were exquisitely sensitive to steroid hormones, becoming highly metastatic when exposed to estradiol. Human high-grade serous ovarian cancer cell lines provide models to study the role of mutant p53 in tumor formation. One hallmark of these HGSOC cells is the presence of polyploidy giant cancer cells (PGCCs) (Figure 3).
Why is ovarian cancer so deadly?
Ovarian cancer is lethal because 1) it is usually detected at a late stage, 2) surgical removal of malignant tissue from the peritoneal cavity is rarely, if ever complete and 3) the cytotoxic drugs used to block ovarian cancer progression routinely lead to drug resistance and persistent tumor growth. The latter is particularly devastating because drug resistance is associated with, and likely mediated by the generation of PGCCs that evade senescence/cell death by a special replication process that gives rise to daughter cells with altered genetic features that confer drug resistance.
1&2) Detection and surgical removal of ovarian cancer
Because ovarian cancers metastasize to the peritoneal cavity, they develop into large structures before they are detected. Although many studies have identified specific markers of ovarian cancer cells in patient serum samples, such as CA125, tumors are large before this method is sensitive enough to be reliable and effective. Therefore, newer technologies are needed to improve the method and sensitivity of detection. Regrettably, surgical removal is risky and never complete.
3) Cytotoxic drugs and PGCCs
The common strategy for reducing ovarian cancer is the administration of cytotoxic drugs, paclitaxel and cisplatin, that target microtubule integrity and thereby impede cell division/ proliferation. Although these drugs are initially effective in causing cancer cell death, the drugs, at the doses that can be safely administered to women, can also cause stress responses in cells that lead to the generation of PGCCs. PGCCs evade senescence/death by entering a specific type of cell replication, known as endoreplication.
Endoreplication normally occurs in early embryonic fetal development and provides cells with the ability to generate daughter cells in which DNA rearrangements occur, leading to genetic diversity. In ovarian cancer cells, endoreplication generates daughter cells that are resistant to the cytotoxic drugs; hence tumor progression continues with cisplatin or paclitaxel treatments. Thus, ovarian cancer research investigators are searching for new therapeutic drugs that will disrupt the endoreplication cycle.
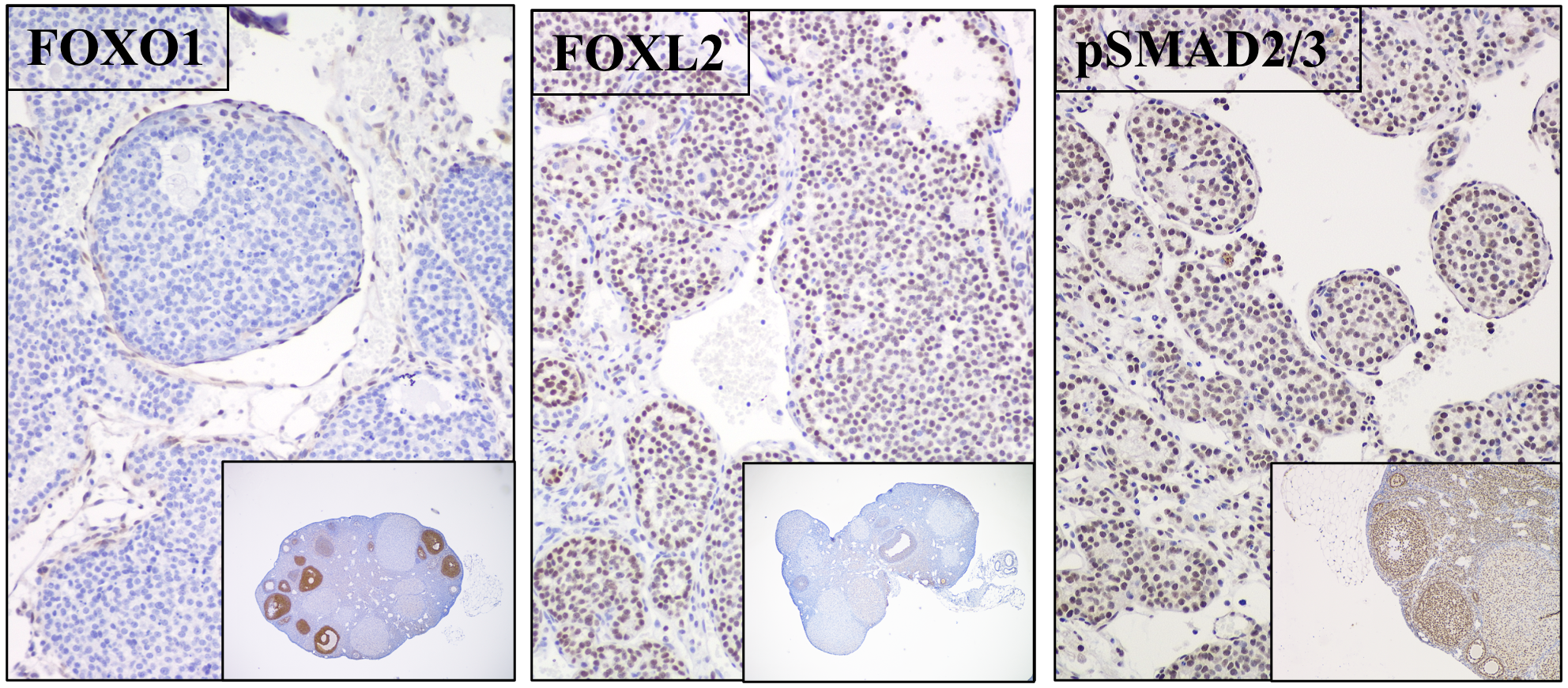
Ovarian granulosa cell tumors
Granulosa cells are rare. Our studies have shown that targeted disruption of Foxo1, Foxo3 and Pten in granulosa cells leads to the formation of granulosa cell tumors that exhibit many characteristics of adult granulosa cell tumors in women and express specific granulosa cell markers (FOXL2/SMAD4); hence they are highly proliferative but retain granulosa cell identity (Figure 4).
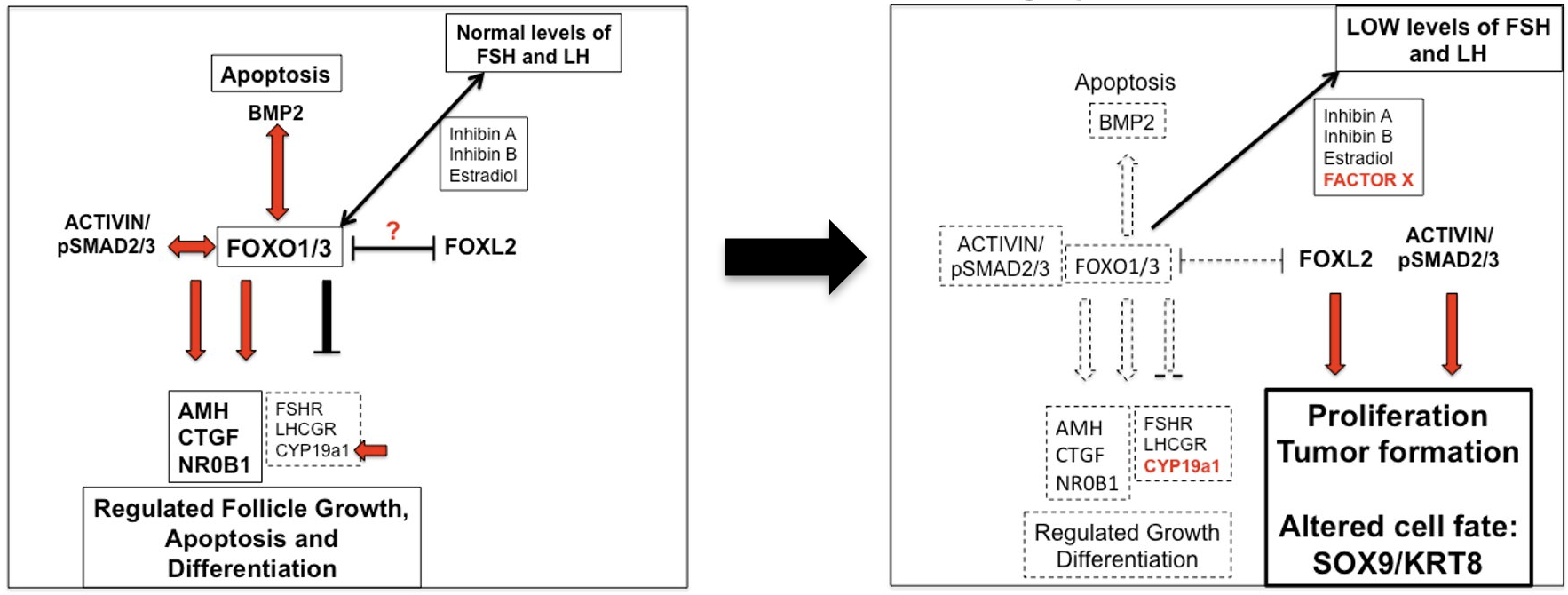
fate decisions in growing follicles
Foxo1/3 depletion alters granulosa cell
fate decisions leading to proliferation and
tumor formation
What does the future hold for ovarian cancer research?
Ovarian cancer is unique concerning cell type and the site of metastasis. However, the underlying events that lead to uncontrolled proliferation are shared with other cancers, such as triple negative breast cancer. As with other cancers, cytotoxic drugs are only partially effective in preventing tumor growth; new therapeutic approaches are needed. Perhaps someday, there will be a novel immune approach or a way to eliminate PGCCs.
References
- Richards JS, Candelaria NR, Lanz RB (2021). Polyploid giant cancer cells and ovarian cancer” new insights into mitotic regulators and polyploidy. Biol Reprod 1-12. https://doi.org/10.1093/biolre/ioab102
- Richards JS (2018). From follicular development to ovarian cancers: An unexpected journey. Vitam Horm 107: 453-472. PMID:2954464

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


