Prof. Michael Schrader at the University of Exeter looks to understand the role of peroxisomes in human health and disease
The proper functioning of eukaryotic cells depends on membrane-bound subcellular compartments (organelles). They create distinct and unique reaction chambers with an optimised environment to promote various metabolic reactions required to sustain life. These compartments are highly dynamic and can alter their position, number, enzymatic content, and morphology according to cellular needs or environmental changes. In addition, organelles interact, communicate, and cooperate in metabolic pathways and tasks distributed across different organelles. Prof. Schrader and his team in the Dept. of Biosciences at the University of Exeter are investigating how these processes are regulated, and what molecular components and mechanisms are involved, with a particular focus on peroxisomes.
Peroxisomes, lipids and organelle cooperation
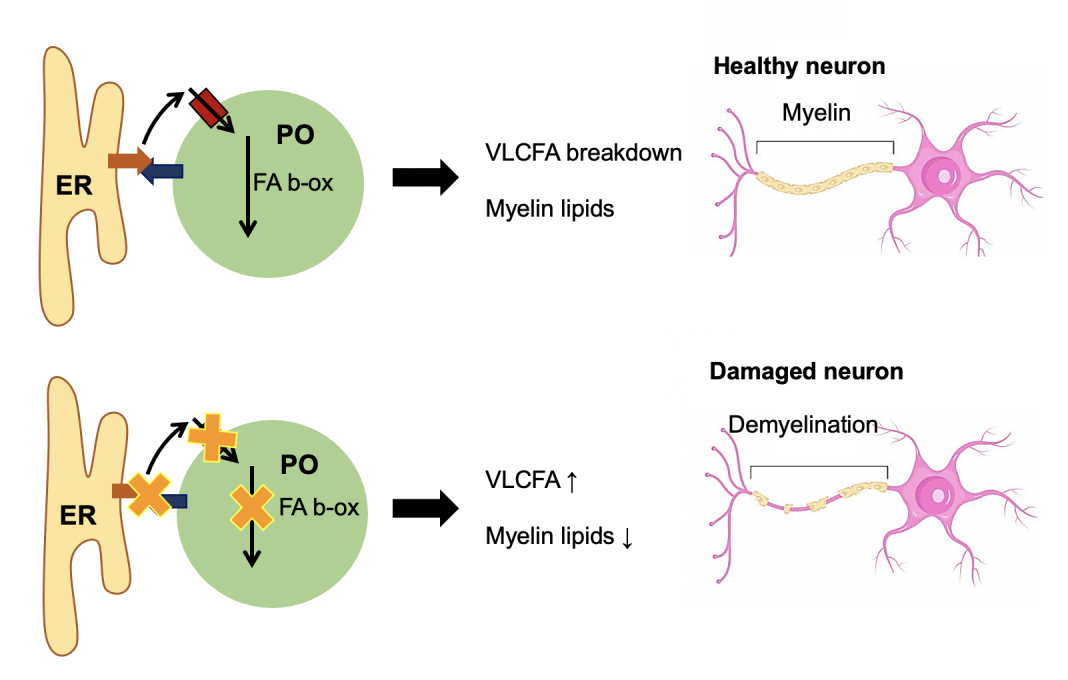
Cells need to process lipids, which serve as an important energy source but also provide essential components for cellular membranes. A central organelle in cellular lipid metabolism is the peroxisome, an understudied yet ubiquitous compartment which is indispensable for human health and development. Important metabolic functions of peroxisomes include the breakdown of fatty acids (in particular very long chain- and other complex fatty acids, which can only be degraded in peroxisomes) and the synthesis of ether lipids, which are important components of cellular membranes (e.g. the insulating myelin sheath of neuronal cells).
Peroxisomes cooperate metabolically with the endoplasmic reticulum (ER),
a large network-like organelle, which is involved in lipid and protein synthesis. Prof. Schrader and his team recently revealed that peroxisomes are in close association with the ER, forming membrane contact sites(1). They discovered that these contacts are important for the delivery of membrane lipids from the ER to peroxisomes, which are required for membrane growth and the formation of new peroxisomes.
Loss of peroxisome function in neurological disorders
Defects in peroxisome function cause an accumulation of complex fatty acids, which can no longer be degraded and a shortage of ether lipids, which are required for the integrity of cellular membranes. This can lead to severe peroxisome-based disorders with neurological abnormalities. The causes for these inherited disorders can lay in the inability of the cells to generate functional peroxisomes due to mutations in peroxisome biogenesis proteins (peroxins).
Peroxins are essential components of the molecular machinery required to import enzymes into the peroxisomal compartment and to generate the peroxisomal membrane. Loss of peroxin function results in the absence of functional peroxisomes and affects all metabolic pathways of the organelle, including fatty acid breakdown and synthesis of ether lipids. This causes a spectrum of progressive and severe peroxisome biogenesis disorders (Zellweger Spectrum Disorders [ZSD], estimated 1/50,000 births) characterised by developmental defects (including brain), the loss of myelin from nerve cells, neurodegeneration, eye problems and deafness. Severe mutations are fatal in childhood.
Another cause for peroxisomal disorders and neurological abnormalities can be mutations in individual peroxisomal enzymes, which affect a specific metabolic pathway rather than the whole organelle. These include defects in enzymes required for the breakdown of fatty acids or for ether lipid synthesis. The most common (estimated 1/14,000 births) is a defect in a peroxisomal membrane transporter (ABCD1), which imports very long chain fatty acids (VLCFA) into peroxisomes. This results in adrenoleukodystrophy (ALD), an inherited disorder affecting the adrenal glands and white matter. The disease is caused by an accumulation of VLCFA, which are no longer degraded in peroxisomes, including in the adrenal gland, which affects hormone production.
The toxic effect on the brain has long been unclear, but recent studies indicate that excess VLCFA can be further elongated by enzymes at the ER and are incorporated into membrane lipids. This likely disturbs membrane organisation with a negative impact on signalling processes and positioning of membrane proteins (ion channels) in neuronal cells, which are required for nerve function(2). In addition, inflammatory processes in the brain can be triggered, leading to the demyelination of nerve cells. The initial ALD symptoms may be mild including behavioural or learning deficits, lack of spatial orientation, and visual disturbances but may progress to loss of communication and voluntary movement, blindness, and ultimately death.
New players in peroxisomal lipid metabolism
Prof. Schrader and his team have recently identified a new fatty acid-binding membrane protein, ACBD5, at peroxisomes, which tethers the organelle to the ER to generate membrane contacts for organelle cooperation (1). Besides its tethering function, ACBD5 appears to recruit VLCFA to peroxisomes to deliver them to the ABCD1 transporter for uptake and degradation in peroxisomes. They and others identified the first patients with a loss of ACBD5 function, who show an accumulation of VLCFA and suffer from leukodystrophy and retinopathy(3). Prof. Schrader and his team are currently investigating the physiological roles of ACBD5 in order to better understand its role in disease. They hypothesize that the dual function of ACBD5 creates a lipid hub at the organelle interface and allows modulation of fatty acid metabolism towards fatty acid elongation or degradation(4).
Improvements in molecular testing and laboratory diagnosis
There are currently no effective treatment options for ZSD, and therapies are mostly supportive, aiming to improve the developmental outcome, survival, and quality of life. However, molecular testing and laboratory diagnosis have improved, and support for children and families is provided by various charities. Prof. Schrader and his team are cooperating with Zellweger UK, a small parent-run charity for families impacted by ZSD.
For ALD, treatment options are limited, but hematopoietic stem cell transplantation has been successfully performed. New hope is coming from gene therapy and the introduction of newborn screening for ALD to enable early detection. Furthermore, drugs are being investigated for their potential to prevent the accumulation of VLCFA to reduce their toxicity. Other approaches aim to identify new compounds which increase the expression of (peroxisomal) genes to either complement the function of the disease gene or stabilise mutated proteins to prevent their degradation and recover or improve lipid import into peroxisomes. Peroxisomal alterations have also
been observed in other neurological disorders such as Alzheimer’s disease. Furthermore, peroxisomes play important roles in the combat of oxidative stress, the regulation of cellular redox balance, as well as in inflammation and immunity. Research to improve understanding of peroxisome biology and the development of approaches to modulate and improve peroxisome function thus have high potential to tackle age-related and neurodegenerative disorders.
References
- Costello et al., ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. 216(2):331-342, 2017. DOI: 10.1083/jcb.201607055
- Kleinecke et al., Peroxisomal dysfunctions cause lysosomal storage and axonal Kv1 channel redistribution in peripheral neuropathy. Elife 4;6:e23332, 2017.
DOI: 10.7554/eLife.23332 - Ferdinandusse et al., ACBD5 deficiency is a disorder of peroxisomal very-long-chain fatty acid metabolism. J. Med. Genet. 54(5):330-337, 2017. DOI: 10.1136/jmedgenet-2016-104132
- Schrader et al., Organelle Interplay – Peroxisome Interactions in Health and Disease. J Inherit Metab Dis. 43(1):71-89, 2020. DOI: 10.1002/jimd.12083

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.