Photodynamic therapy can stimulate a person’s own immune system to better recognise – and fight – cancer tumours, say Mary Potasek, PhD and Karl Beeson, PhD of Simphotek and Theresa M Busch, PhD of the Department of Radiation Oncology, University of Pennsylvania
Our immune system is central to our health because it recognises microorganisms such as bacteria, viruses, parasites and fungi that are foreign to our body and defends us against them. In the case of cancer, our body’s own cells have changed (mutated), but if successfully detected by our immune system as different from the originating normal cells, the proliferation of cancer cells and growth of a tumour can be controlled.
Toward this goal, cancer therapy may strive to make cancer cells more visible to a person’s immune system. Alternatively, or additionally, cancer therapies may act to magnify the response of immune cells against the tumour. In this way, a person’s own immune system can be induced to recognise and fight a cancer. Tumours that succeed in growing uncontrollably have evaded detection and/or destruction by immune cells.
Photodynamic therapy: How it works
Photodynamic therapy (PDT) is a multicomponent therapy based in the activation of a molecular photosensitiser (PS) that is allowed to accumulate in diseased tissue such as a tumour. Generally, a photosensitizer is activated by light from a visible or near-infrared light source (often a laser). This light can be delivered to the tumor by multiple means, including through lens-tipped optical fibers that illuminate the tumor from its surface or cylindrical diffuser fibers that are inserted into the tumor and illuminate it from within.
Upon its activation, the PS forms cytotoxic molecules, including reactive oxygen species that irreversibly damage cells, leading to apoptosis and necrosis, among other cell death mechanisms. Dying cells can stimulate inflammation and an immune response. Thus, PDT can stimulate immune recognition of tumour cells and promote an anti-tumour immune response.
In conjunction with specific indications, PDT is FDA-approved for cancers or precancers that include non-small cell lung cancer, Barrett oesophagus, oesophageal cancer, actinic keratosis, basal cell carcinoma, squamous cell carcinoma and cutaneous T-cell lymphoma. In clinical trials, PDT is studied in treatment of cancers of the lung, breast, brain, pancreas, prostate and head and neck, among other organ sites.
Innate and adaptive immunity
The major arms of the immune system include innate immunity and adaptive immunity. Innate immunity is composed of an early and non-specific response to an immunological threat. For example, the swelling and redness associated with inflammation is part of the innate immune response and involves cells of the innate immune system, such as monocytes, macrophages, neutrophils, dendritic cells and natural killer cells. Many of these cells are phagocytes, that is, they will “eat” (phagocytize) other cells or material in an effort to protect the body.
Initiation of an innate immune response can help to produce adaptive immunity. In an adaptive immune response, there is specific recognition of the particular foreign material (antigen) to direct the cytotoxic action of the immune system. It can lead to immunological memory of the material as foreign, facilitating combat against its presence in another site of the body or at a later time. Lymphocytes are cells of the adaptive immune system, including T-cells, B-cells and subpopulations of these cell types.
PDT-generated immunity
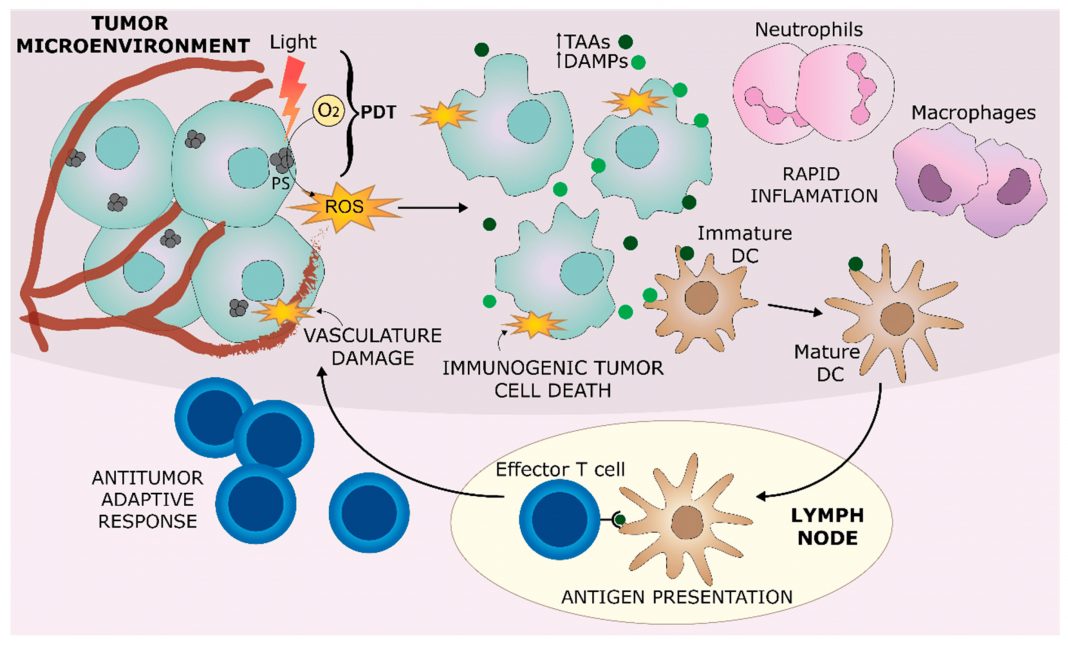
The role of PDT in triggering an immune response in the tumour microenvironment is summarised in Figure 1 from Hernandez, et al (1).
Upon its activation by light, PS leads to the production of ROS, which directly damages tumour cells, as well as blood vessels that are supporting the tumour. Localised inflammation can rapidly develop in the tumour, characterised by the accumulation and action of myeloid cells, such as neutrophils and macrophages. DAMPs are released from dying cells, alongside the exposure of TAAs in a process known as immunogenic tumour cell death. Antigen presenting cells, such as DCs, are stimulated by DAMPs within the inflammatory environment, leading them to capture TAAs and present them to effector T-cells in nearby lymph nodes. This process can lead to the development of antitumor adaptive immunity.
Immunotherapy and clinical trial combinations with PDT
Immunotherapy is a treatment modality that acts to promote a person’s own immune system to fight cancer. Due to PDT’s ability to generate immunogenic tumour cell death, it may cooperate with immunotherapy to improve treatment outcomes.
Immune checkpoint inhibitors (ICIs) are one type of immunotherapy drug successfully employed in treatment of several types of cancers. ICIs work as antibodies that target proteins on immune cells known as checkpoint molecules because they promote or suppress immune responses. The receptor CTLA-4 and its ligands CD80/CD86 constitute one immune checkpoint pathway. CTLA-4 receptor on T-cells will negatively regulate T-cell function when bound to CD80/CD86, expressed, eg on DCs.
Similarly, in another immune checkpoint pathway, T-cell activity is negatively regulated by the binding of PD-L1/PD-L2 ligands on tumour cells and/or other cell types (eg myeloid cells) to PD-1 receptors that are expressed in a context-dependent manner on activated T- and other immune cells. (2)
The expression of immune checkpoint ligands can define an immune suppressive function of macrophages or neutrophils – characterising them as monocytic myeloid-derived suppressor cells (M-MDSCs) or granulocytic myeloid-derived suppressor cells (G-MDSCs). Immune checkpoint pathways can suppress T-cell activity in the tumour itself.
Moreover, in the lymph nodes, immune checkpoint pathways can control tumour antigen presentation by DCs to receptors on T-cells and/or inhibit the proliferation of antigen-experienced T- cells, thereby impeding the initiation of a productive T-cell response.
The addition of ICIs to PDT has been studied preclinically, producing favourable results that drive interest in clinical trials combining these modalities (3). As of 11/1/23, clinicaltrials.gov reports on three clinical trials expressly evaluating combinations of PDT with an ICI. NCT04836429, NCT05386056 and NCT04400539 are being conducted in patients with non-small cell lung cancer with pleural disease, metastatic oesophageal squamous cell carcinoma and malignant pleural mesothelioma, respectively.
Conclusion
PDT can be highly effective in generating immunogenic cell death that promotes T-cell mediated immunity against the treated tumour. An anti-tumour immune response can be augmented by the addition of ICIs, which act to combat immunosuppression in the tumour environment. This promising combination is actively being investigated in clinical trials.
Disclosures
TMB reports equity from Simphotek, Inc and personal fees from Lumeda, Inc and Ion Beam Applications S.A. (IBA), outside of the scope of this work.
References
- B. Hernandez, et. al. “Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations”, J. Clin. Med. 2020, 9, 333; https://doi.org/10.3390/jcm9020333.
- G.M. Cramer, et. al. “Photodynamic Therapy and Immune Checkpoint Blockade”, Photochem Photobiol. 2020, 96(5):954-961. https://doi.org/10.1111/php.13300.
- S. Anand, et.al. “Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition”, Pharmaceuticals. 2021, 14, 447; https://doi.org/10.3390/ph14050447.