Martin Schuettler, Chief Technology Officer at CorTec GmbH, addresses key questions around the development of the company’s innovative Brain Interchange System to support stroke rehabilitation therapy
CorTec has developed the Brain Interchange (BIC) implant system to improve stroke rehabilitation outcomes by enhancing the effect of conventional physio and/or occupational therapy. (1, 2) The effectiveness of the new implant-supported therapy will be investigated in an early feasibility study planned for 2024 to be carried out in Washington, US.
While working on the approval of the feasibility study, uncertainties and unknowns of the study are investigated. These involve questions around usability and technical performance.
Usability aspects
Neurologists, clinical researchers, occupational therapists, and reimbursement experts were interviewed to identify potential gains and pain points for using the BIC system in conjunction with conventional therapy to treat upper extremity functional deficits. Usability is a major topic; as long as the system is easy to activate, does not require calibration or other expert interaction, and is not physically impeding exercises during rehabilitation sessions, the patient and therapist are expected to accept it. However, the surgery required for applying the system and removing it after nine to 12 months must be justified by a substantial functional improvement compared to conventional therapy. Today, it is presumed that an improvement of seven or more points in the Upper Extremity Fugl-Meyer (UEFM) score would be considered substantial and justify an implantation for patients between 25 and 48 UEFM.
The early feasibility study is an instrument to provide input into a device development that would otherwise not be accessible. The patients (four to 12) are carefully guided and supervised; hence, not all requirements essential for the successful commercialization of an implant system must be met; aspects that are cared for by the clinical team, who work closely with the patient, can be in a premature state. In some cases, this applies to aspects of usability.

Functional testing and validations
Regarding system functionality, very few or no open questions should remain before considering implantation in the framework of an EFS. All questions that can be answered before were addressed either in bench tests or pre-clinical studies. While the latter was used to investigate device longevity, general device function and biocompatibility of implanted hardware, bench testing permits the validation of system performance, mechanical robustness, electromagnetic compliance, electrical safety, cybersecurity, the effectiveness of built-in safety measures, sterilization effectiveness, and more. These tests are carried out by the medical device manufacturer and accredited laboratories and are, to a high degree, standardized by international standards.
Performance data from humans before clinical study
Interaction of the implant system with the brain is difficult to accurately model on the bench. This is done to a certain extent using a saline basin representing the brain’s electrical volume conduction and playing brain signals recorded in different settings back to this basin while using the implant to record from and stimulate to the saline; however, the conclusion to be drawn for this setup is still limited.
An alternative to these studies in vitro is the involvement of patients who undergo sub-chronic brain mapping. These patients, typically scheduled for a surgical brain procedure, have electrodes implanted for a few days up to a few weeks and are cared for in an epilepsy monitoring unit. The leads of the implanted electrodes are externalized and accessible for connection to electrical recorders and stimulators. Patients who formally consent to participate in an experimental session (besides their diagnostic protocol) provide an opportunity to collect in vivo data. Such experimental sessions, in case they are of minor risk to the patient, need approval by the local ethics committee and do not require approval by the FDA (US) or Competent Authorities (EU).
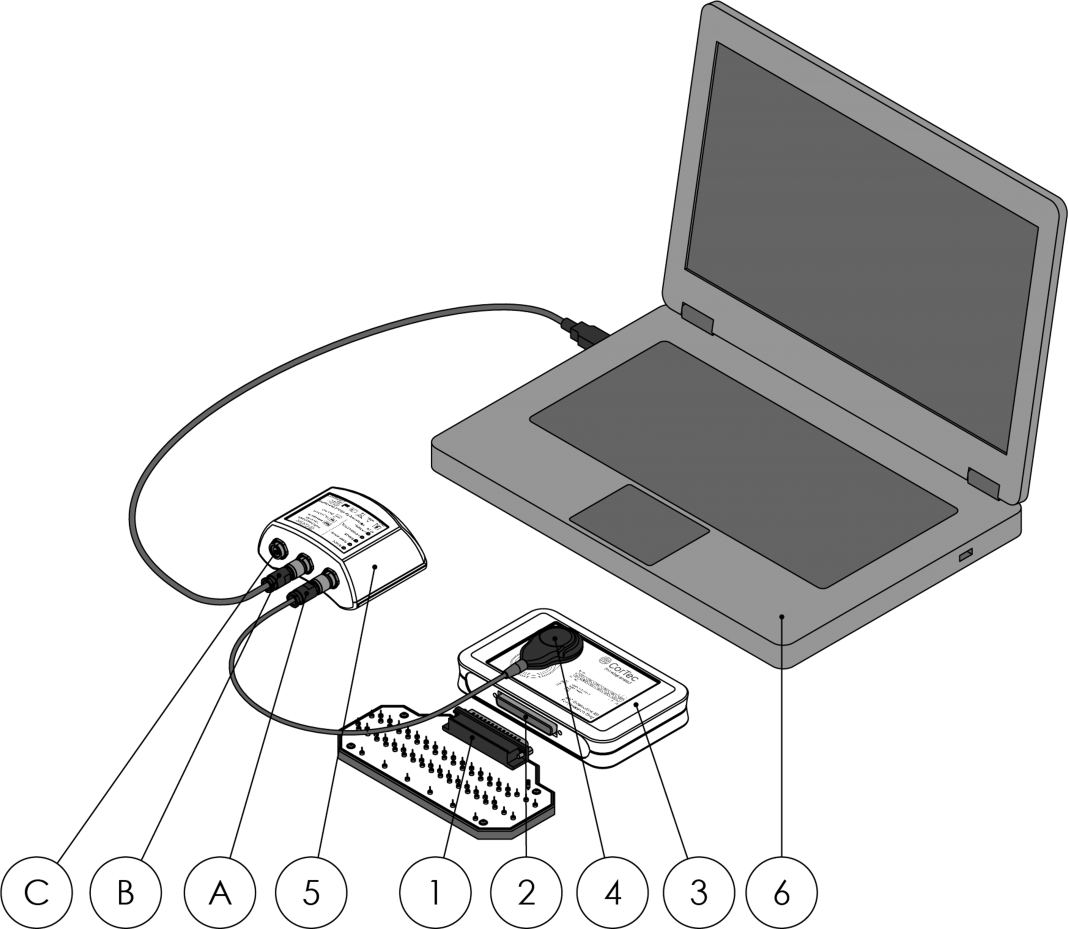
In preparation for implant studies, also suitable for experimental sessions in the epilepsy monitoring unit, CorTec developed a version of the implant system that is easy to interface with on the bench / in the monitoring unit and meant for evaluation of system performance and for getting acquainted with system programming. Hence, it is called the ‘BIC Evaluation Kit.’
The evaluation kit is an implant in an implant module box with an electrode connector instead of brain electrodes attached to it. The electrode model module can be connected to the electrode connector, an electrical representation of electrodes placed in the brain. Using laboratory equipment such as signal sources and oscilloscopes, the function of the implant system can be observed – by feeding in small electrical signals, mimicking brain activity, (3) or watching electrical pulses sent by the implant into the electrode model, for example.
Once the programming of the system and the resulting performance are evaluated, the system can be connected to the externalized leads of a patient, disconnecting the electrode model module. Now, studies of human brain recording and stimulation can be carried out within the scope of the session plan.
In the past, CorTec had the chance to benefit from this methodology in the US in the field of epilepsy treatment research (4), showing that the wireless implant system has epilepsy onset detection qualities comparable to that of established clinical amplifiers and stroke rehabilitation research which started by the end of 2023 with the closed-loop stimulation session with promising results.
Conclusion
The BIC Evaluation Kit permits collecting human data in vivo as an important preparation and risk mitigation for the early feasibility study, using a fully implanted system, planned for 2024.
References
- Schuettler, M. “Options for stroke survivors: From stroke to conventional therapy”. Open Access Government, 4 Oct 2023a.
- Schuettler, M. “CorTec’s Brain Interchange System: Revolutionizing brain therapy with closed-loop neuromodulation”. Open Access Government, 4 July 2023b.
- Cho, H., Ojemann, J., and Herron, J.. “Open Mind Neuromodulation Interface for the CorTec Brain Interchange (OMNI-BIC): an investigational distributed research platform for next-generation clinical neuromodulation research.” 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER). I EEE, 2023.
- Ayyoubi, A. H., Besheli, B. F., Quach, M. M., Gavvala, J. R., Goldman, A. M., Swamy, C. P., Bartoli, E., Adkinson, J., Curry, D. J., Sheth, S. A., Francis, D. J., Ince, N. F. “The Early Feasibility of Recording Prolonged iEEG in Human Subjects Using CorTec Brain Interchange System: Assessment of Signal Quality, System Performance, and Spike Analysis.” Nature Scientific Reports, 2023 – SUBMITTED

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.