Deborah McElhone, Head of Sustainability (Pharma) from CPI, argues that process intensification is a game changer for the pharma market
Pharmaceutical manufacturing is a competitive field, with patient demand driving the push for more sustainable products. Enter process intensification (PI) – a transformative approach that promises to revolutionise the pharma market.
PI strategies are becoming more prevalent in this ever-evolving landscape, and its core focuses on redesigning production processes, often employing innovative techniques to streamline manufacturing. The goal? To make medicine faster, cheaper, and more sustainable for the planet. Several aspects are central to this approach:
- Flexible and modular manufacturing:
- Including the adoption of continuous manufacturing and use of microreactors
- Process analytical technologies (PAT).
- Approach to waste minimisation: – Green chemistry.
- Energy efficiency.
- Circular economy principles.
- Utilising single-use technologies.
- Implementing digital tools and automation
Process intensification is all about achieving more with less. In the pharma industry, this can happen in two ways: process unit intensification and plant intensification. PI benefits both the industry and the environment.
What sustainable advancements are making an impact? Flexible manufacturing, i.e. continuous technology
A promising aspect of PI is the shift from traditional batch processing to continuous manufacturing, whether this includes using flow reactors for intermediate and/or API production or continuous, direct compression for tablets. Continuous technology operates nonstop, allowing for better control over the reaction conditions due to steady production rates. With small volumes and improved heat and mass transfer capabilities, continuous flow reactors can enhance safety by enabling reactions that are not feasible with traditional batch methods. Novel APIs could be manufactured that were previously inconceivable.
The sustainability benefits of continuous manufacturing are significant. Reduced energy consumption, raw material usage, and waste generation contribute to lower greenhouse gas emissions and a smaller environmental impact. The productivity gains from continuous manufacturing also translate into business cost savings. For example, an Oral Solid Dose case study has demonstrated a capex reduction of 20-76% and an operational saving of 9-40%, depending on API load. (1)
To support continuous technology, PAT, once seen as a cutting-edge innovation, has now become a standard expectation in the industry. It plays a crucial role by providing real-time monitoring capability. By integrating sensors and automation control systems, in line with the process, advanced PAT can provide continuous feedback, enabling real-time process adjustments to be made that will ensure the product quality remains within specification during production, reducing the risk of deviations.
Continuous technology is constantly advancing in the pharma industry. It is a dynamic field with exciting new possibilities. Keep an eye on this space!
Waste minimisation
Concerning PI, waste minimisation covers a variety of topics. It includes green chemistry and engineering practices such as recycling waste as feedstock and recovering valuable materials, i.e. solvents, catalysts, heat, and water for reuse (critical in areas of water scarcity). Catalyst recovery and process optimisation are also important subtopics as they reduce the need to mine precious metals, which can have a significant environmental impact.
In the pharmaceutical industry, strict regulations limit the extent of recycling due to contamination concerns. However, ongoing innovations are addressing these limitations, and by continuing to focus on waste minimisation efforts, the potential of PI can be realised further.
Resource-intensive tasks such as cleaning and verification analytics can be mitigated by disposable components, reducing downtime between production runs. While disposable items can seem counterintuitive with waste minimisation and circular economy practices, single-use systems are rising. Single-use technologies are especially beneficial in biopharmaceutical manufacturing, where flexibility and quick product changeover are essential. The critical focus now is understanding how recycling, recovery, and reuse can be integrated into a single-use strategy.
By following these principles, the pharmaceutical industry can design processes from fundamentally less wasteful concepts.
Go digital
To further intensify processes and ensure sustainable practices, advanced algorithms and control systems are being developed to manage the complexities. Digital twin and modelling technology optimise reactions, improve yields and reduce waste. (2)
Automation technologies are also worth mentioning. This includes everything from artificial intelligence (AI) and machine learning (ML), PAT for monitoring, SCADA control, robotics and collaborative robots (cobots) and data acquisition. The immense value of data has been fully recognised and is now being harnessed to uncover valuable insights into manufacturing processes. This new knowledge can truly unlock the potential of digital.
Regulatory framework
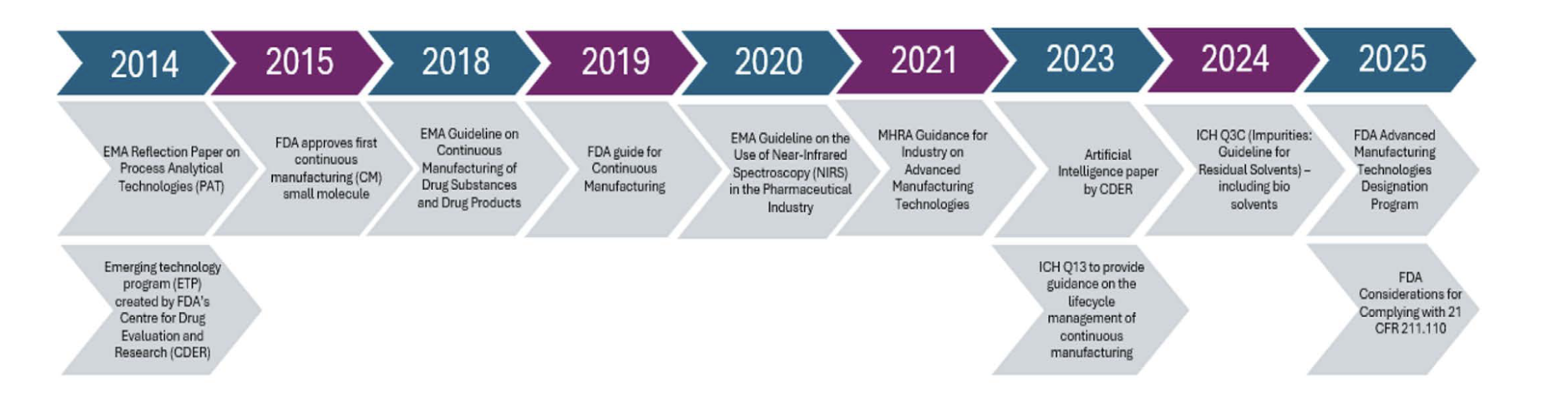
We cannot conclude the discussion without acknowledging the influence of PI technology on the current pharmaceutical regulatory framework. Incredible resources have been allocated to show support and encourage the adoption of more sustainable manufacturing practices through the publication of guidelines and incentives (see Figure 1).
Our future outlook
PI, driven by continuous manufacturing and advanced technologies, is a game changer for the pharmaceutical market, significantly supporting the industry’s sustainability commitment. We must embrace this future and embed sustainability into everything that we do.


