Here Tryp Therapeutics examine the viability of using psychedelic therapies for Binge Eating Disorder and the potential results that using psychedelic-assisted psychotherapy could have on different eating disorders
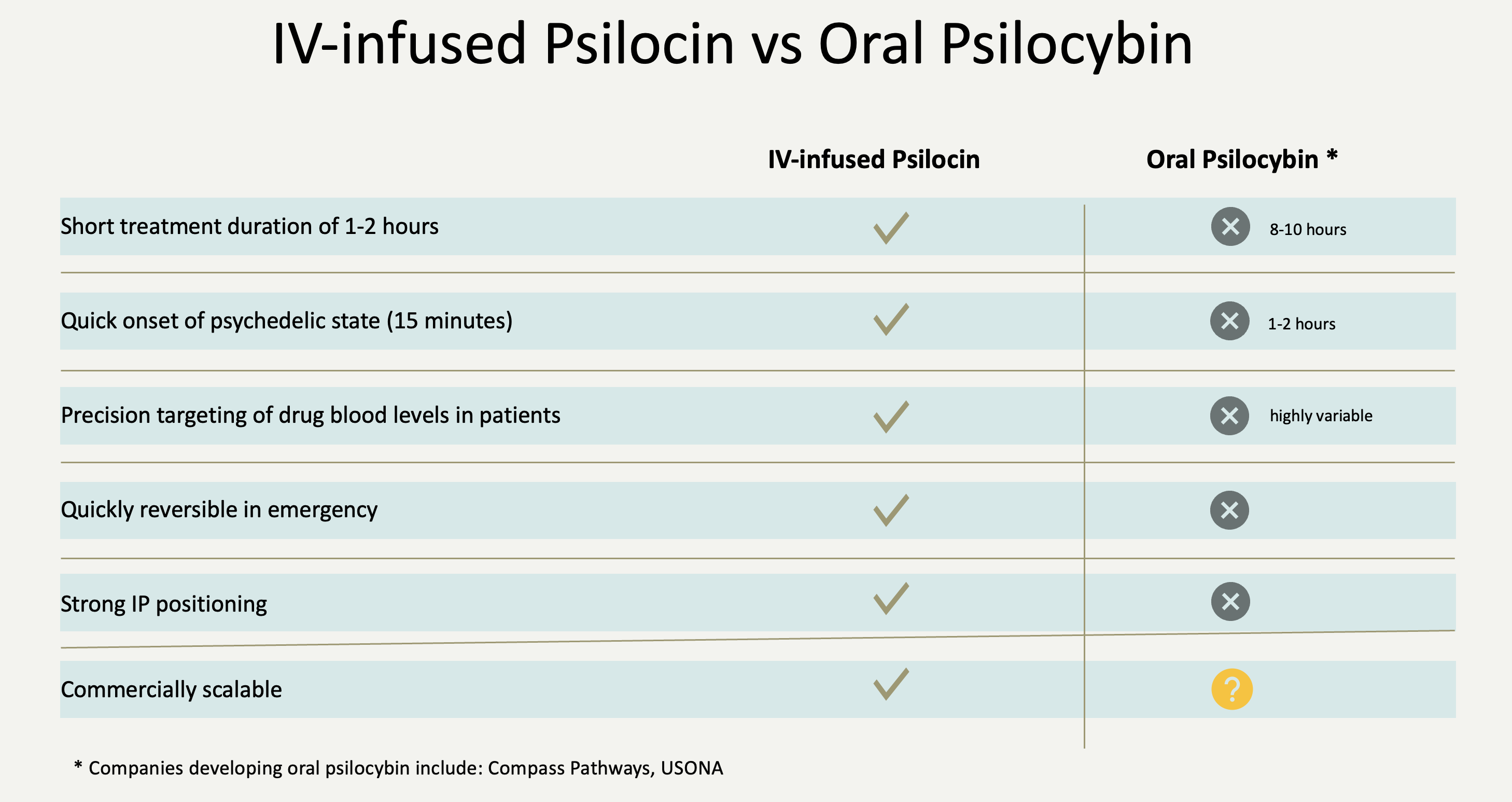
Tryp Therapeutics is a clinical-stage biotechnology company dedicated to the development of exclusive and innovative formulations for administering psilocin in conjunction with psychotherapy, aiming to address unmet medical needs in various diseases. Our primary focus is on our lead program, TRP-8803, which represents a proprietary formulation of intravenous (IV) infused psilocin, the active metabolite of psilocybin.
This formulation overcomes several limitations associated with oral psilocybin administration. By directly administering psilocin via the intravenous route, we achieve the most precise form of dosing, ensuring the reproducibility of blood levels necessary for clinical efficacy while preserving safety. In contrast, oral administration of psilocybin relies on enzymatic conversion of psilocybin to psilocin, resulting in substantial variability in drug blood levels among patients, potentially confounding the planned outcome of clinical studies.
Leveraging psychedelic therapies for Binge Eating Disorder
TRP-8803 addresses additional limitations associated with orally administered psilocybin, including the time to onset of the psychedelic experience (1-2 hours) and the duration of the experience (6-8 hours).
A treatment protocol utilizing oral psilocybin will be challenging to scale commercially due to the extensive time commitment required by both the patient and therapists.
In contrast, TRP-8803 enables a relatively rapid induction of the psychedelic experience within 10-15 minutes while providing control over the depth and duration of the experience. This reduction in treatment time to a manageable 1-2 hours represents a significant advantage over orally administered psilocybin. And the ability to achieve precision targeting of drug blood levels is likely to provide significant efficacy advantages as well.
Tryp Therapeutics is concurrently identifying novel indications for psychedelic-assisted psychotherapy via its oral psilocybin program, TRP-8802. Where a preliminary clinical benefit is demonstrated, subsequent studies are expected to utilize TRP-8803 (IV-infused psilocin).
These small pilot studies offer a cost-effective approach to identifying signals for new indications whilst expediting and mitigating risks in the drug development process. The resulting data contributes to the establishment of novel intellectual property claims and assists in optimizing psychotherapy protocols for each respective indication.
Collaborating with esteemed institutions such as the University of Florida, the University of Michigan, and Harvard Medical School, Tryp’s clinical focus with TRP-8802 is on eating disorders and chronic pain, including fibromyalgia and abdominal pain associated with Intestinal bowel Syndrome (IBS).
Psychotherapy, BED, anxiety and depression
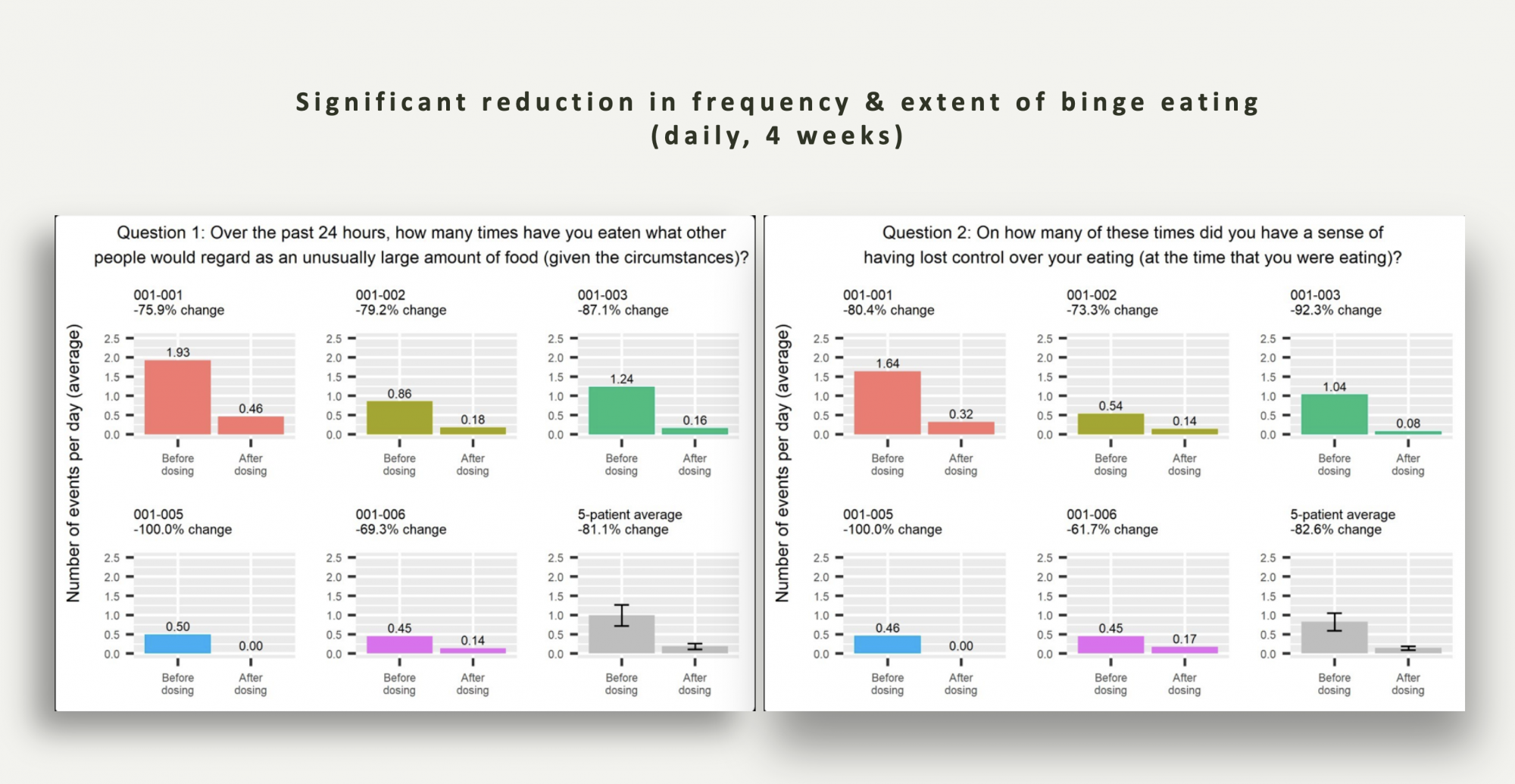
Tryp recently published the interim results from an eating disorder study conducted at the University of Florida in patients diagnosed with binge eating disorder (BED). These patients were enrolled in a 4-week run-in period, during which time patients were required to maintain a daily diary on their smartphones to record the number of binge eating episodes and their perception of losing control over their eating habits.
Additionally, they completed questionnaires assessing anxiety and depression. The first two weeks served as a baseline data collection period, while the subsequent two weeks involved 8 hours of psychotherapy administered by two trained psychotherapists, along with EEG and fMRI neurological evaluations. After the 4-week run-in period, patients received oral psilocybin (25 mg capsule) administered by one of the psychotherapists in a setting that met established set and setting requirements.
The following morning, patients returned to the clinic for an integration session with their therapists. The primary clinical endpoint, as agreed upon with the FDA, was the number of daily binge eating episodes four weeks after drug administration.
The results for the number of daily binge eating episodes and the frequency of perceived loss of control over eating behaviour are presented in the graphs above. These findings reveal a remarkable reduction of over 80% in both the number of daily binge eating episodes and the instances of perceived loss of control over eating behaviour compared to baseline following psilocybin administration.
Importantly, continued monitoring of patients for up to 60 days demonstrated the durability of the effect of the treatment. These results provide substantial support and validation for the clinical potential of psychedelic-assisted psychotherapy to exert a meaningful clinical impact on eating behavior in patients with BED.
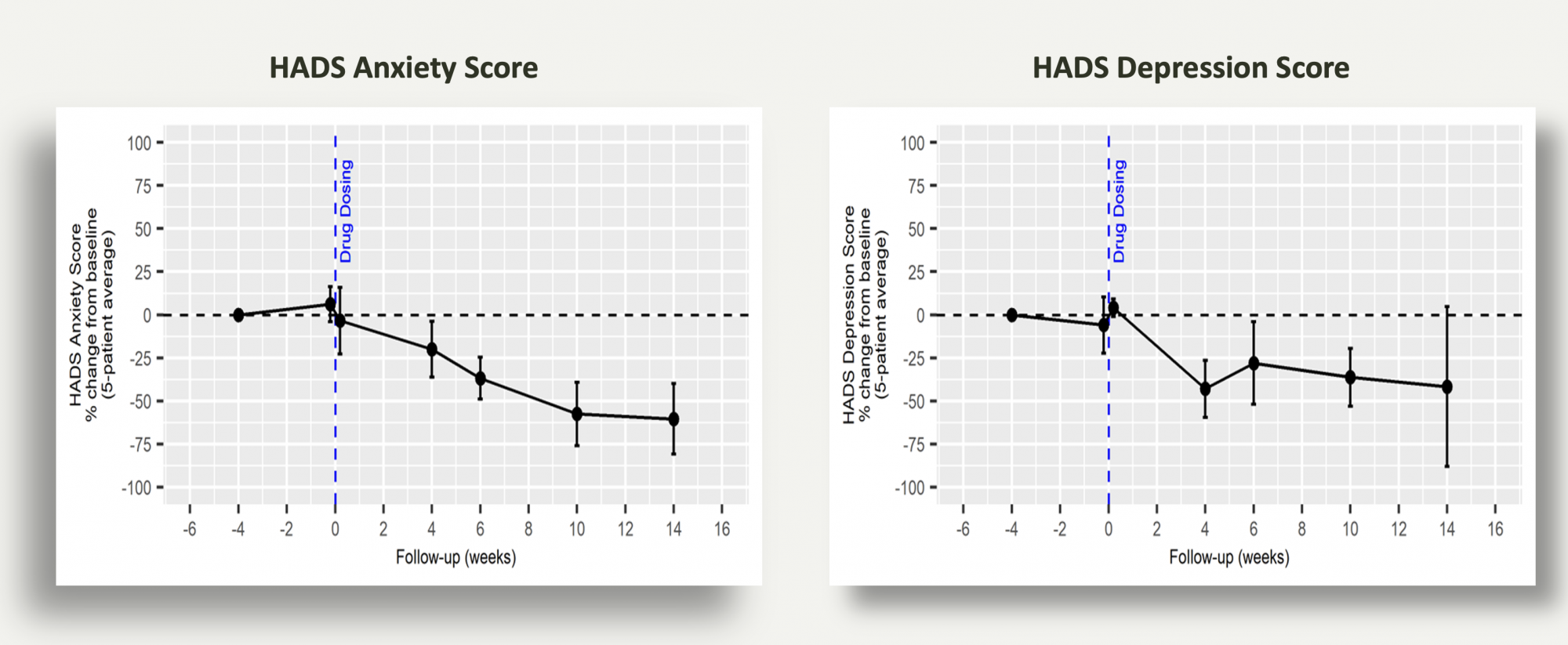
Patients with BED also frequently experience symptoms of anxiety and depression. During the 4-week run-in period, patients completed the Hospital Anxiety and Depression Scale (HADS) questionnaire to assess anxiety and depression levels. This assessment was repeated during the subsequent 4-week period following psilocybin administration. The results are depicted in the graphic below.
These findings demonstrate that psychedelic-assisted psychotherapy led to improvements in both anxiety and depression scores among these patients, aligning with previously published results in patients with treatment-resistant depression (TRD) and anxiety disorders.
The promising potential of psychedelic-assisted psychotherapy
The interim results from Tryp’s Binge Eating Phase 2a highlight the promising potential of psychedelic therapies for Binge Eating Disorder and, potentially, other eating disorders. Looking ahead, future studies hold even greater promise with the utilization of TRP-8803, an intravenous (IV) infusion of psilocin with its advantages of rapid onset, precise dosing, and control over the depth and duration of the psychedelic experience.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


