Flipons are the next step in DNA research. What they are, their role in DNA and RNA coding, their impact on medical science, and their relation to the immune system are discussed here
Flipons are DNA and RNA sequences that adopt alternative forms inside our bodies. These different ways of folding nucleic acids were first discovered after Watson and Crick proposed their famous model for DNA called the ‘secret of life’.
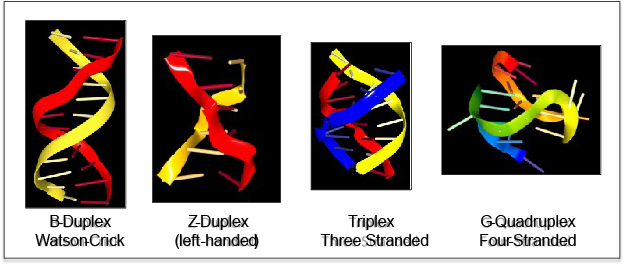
They were considered mere curiosities until the first crystal structure of DNA revealed that the DNA helix was wound in a left-handed direction rather than the expected right-handed twist of B-DNA.
This form of DNA was called Z-DNA because of its zig-zag backbone. The surprise was immense and set experimenters off in a frenzy to find a role for Z-DNA in genetics. Other unusual structures were later discovered – of three-or four-strand DNA, rather than two, used for B-DNA and Z-DNA. Unfortunately, as early investigations were met with failure, interest in anything other than B-DNA and funding for research began to wane.
However, the few pioneers who persisted were rewarded with major discoveries that changed how we think genetic information is stored in DNA.
How do flipons work?
The modern roles of Z-flipons (meaning those that form either Z-RNA or Z-DNA) were recently revealed. The first step was to see whether any protein at all bonded to Z-DNA. This task was not as easy as it sounds.
Like everything else, DNA prefers a lower energy state. As Z-DNA is a higher energy form than B-DNA, making a stable Z-DNA probe was difficult, and finding proteins that recognized Z-DNA was challenging.
Without finding them, it was also impossible to know whether they were specific to a particular Z-DNA sequence or the tissues in which they were present. The solution to this problem involved chemically modified DNA that could form Z-DNA under normal salt conditions.
The work progressed rapidly, and the first Z-DNA-specific protein was found. Again, there was another surprise. The protein was specific for the structure and not for any Z-DNA sequence. It also similarly bound Z-RNA. So, what did the protein do? The protein edited the RNA to replace the adenosine base with another base read differently during the protein synthesis. It could cause the recoding of the protein made without any DNA mutation involved.
Although it was quickly shown that this editing had no role in changing the development of mice or humans, it led to a tool under development to correct disease-causing variants in humans.
Many consider this approach safer than using the CRISPR enzymes to change the DNA irreversibly. With RNA editing, the changes can be long-lasting but not permanent.
Flipons and the immune response
What, then, was the purpose of the RNA editing enzyme if it was not needed in development? The enzyme was part of a system to defend against viruses. Most importantly, the enzyme was involved in turning interferon responses off.
This role was confirmed in families where the enzyme had a sequence variation that impaired its function. Children develop an inflammatory condition marked by high interferon levels and brain calcification, which can lead to early death. While most variants diminished enzyme activity, two were in the Z-DNA/Z-RNA binding domain.
A careful study of the families afflicted proved the first evidence of a biological role for this left-handed conformation.
Z-DNA or Z-RNA and an anti-viral interferon response
To stay healthy, interferon responses against self-RNAs must be avoided. It turns out that many RNAs also carry with them repeat elements (RE) that populate the genome early on. They would copy themselves into RNA and then paste themselves back into DNA at another location. The REs were a threat as they tended to insert themselves into active genes; editing the RE RNA was one way of disrupting their spread.
But as often happens, these attackers were stopped and used for a different purpose. They now identify transcripts made by the host, not by a virus. They also contain a Z-box that will form Z-RNA, allowing the editing enzyme to recognize them and stop the interferon response against self-RNAs from amplifying.
The second part is that there is another Z-DNA and Z-RNA binding protein in the human genome related to the editing enzyme that causes cell death. If the editing enzyme fails to stop the interferon response or prevent viruses from replicating, this enzyme will step in and kill the cell.
Often this response is highly inflammatory and helps bolster immunity against a virus. These roles for both proteins are relatively recent. They played important parts in the pandemics occurring as cities were forming. Both smallpox and measles mortality reflected the battle fought with these two Z-DNA and Z-RNA binding proteins. We have shown how small molecules that activate similar responses can be used to treat cancer by enhancing immune responses against malignant cells.
What’s next for flipons?
Flipons are a form of binary genetic encoding.
They are either in one conformation or another. By using structure-specific proteins to recognize each shape, assembling different complexes to change how information is interpreted from the genome is possible. The energy needed to alter flipon confirmation comes from many sources, such as that released by polymerases when they make RNA.
The different types of flipons play unique roles in this process. Z-flipons store the energy to reset a gene so that a new round of transcription can occur. Three-stranded structures help anchor various machinery at a particular site in a gene. Four-stranded structures tell the machinery to start working.
Flipons are sequences that are prone to open up and expose single-stranded DNA bases as they flip from one conformation to another. At that stage they can bind small RNAs. The RNA can then regulate flipons in a sequence-specific manner. None of these steps require the highly evolved sequence-specific proteins that were the major focus after the Watson and Crick model was proposed.
The RNAs can be swapped out rather rapidly and enable faster evolutionary changes than it is possible to make a built-for-purpose protein to do the same job. Already small synthetic RNAs are deployed in the clinic to dispose of troublesome RNAs that cause disease. Patients are treated twice a year.
This success story is only the tip of the iceberg when it comes to RNA therapeutics. Using small RNAs to target flipon conformation offers exciting new therapeutic possibilities that are now only being explored. Despite its potential, this innovative field of science is woefully underfunded.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.