Brian Tait, Chief Scientific Officer at Haplomic Technologies Pty Ltd, examines single chromosome sequencing to obtain genetic phase (haplotyping)
Proteins are coded for by genes in a cis transfiguration. When a gene has two or more polymorphisms, unless the genetic phase is established definitively, it is not possible to assign protein sequences. Currently, mathematical approaches are used to assign a probability of phase, and long-range sequencing addresses this issue. However, neither technique is 100% accurate, and there are other factors with both approaches that cast doubt on their reliability as long-term answers to phasing.
The mathematical approaches to haplotyping rely on a phenomenon called linkage disequilibrium (LD), which occurs when two neighbouring single nucleotide polymorphisms (SNPs) co-occur with a frequency that is statistically significantly different from equilibrium. For example, if two neighbouring SNPs occur with a frequency of 50% in a population, then at equilibrium, the co-occurrence of both SNPs should be a product of their individual frequencies, i.e. 50×50= 25%.
Any statistically significant deviation from this frequency indicates LD, which can be either positive or negative. LD is a common feature across the genome and has been used to indicate SNP haplotypes. However, LD fails the test of reliability when disease patients are being studied or when non-Caucasian racial groups are studied.
The only method that gives 100% accuracy apart from nuclear family studies, in haplotype calling, is individual chromosome sequencing, which has not been reported to date.
The value of haplotyping
The value of haplotyping, also called genetic phasing, has been a fundamental feature of the HLA system for over 60 years. The gold standard for establishing phase, until recently, has been family studies. Unfortunately, many patients do not have access to the minimum number of family members required to establish phase.
The archetypal example of the value of phasing is bone marrow transplantation, where it has been shown that HLA genetically unrelated recipient/donor pairs who are allele matched at the HLA locus but in a different phase, have inferior outcomes to recipient/donor pairs where haplotype matching can be demonstrated. (Kitcharoen, 2006, Petersdorf 2007, Maskalana, 2020).
Multiple other examples of phasing being important include polymorphisms in the CYP family of genes influencing drug metabolism and immunomodulatory approaches to cancer treatment, such as checkpoint inhibition.
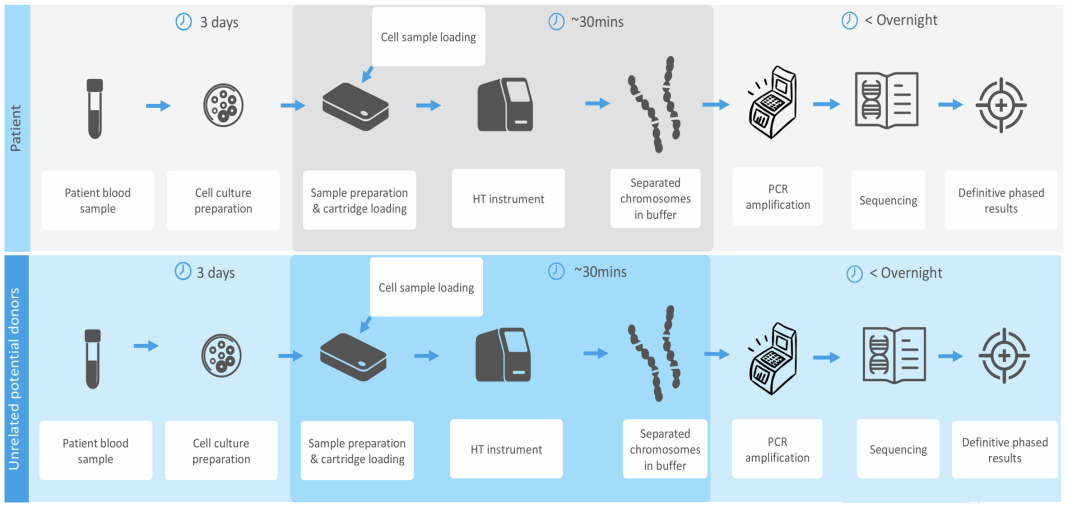
Haplomic Technologies (HT) has been focussed for the last 10 years on a reliable and routine method for haplotyping. In collaboration with the University of Adelaide, we have adapted the FISH technique (fluorescent in situ hybridisation) for the labelling of chromosomes using centromeric probes. We have concentrated initially on chromosome 6 to assign HLA haplotypes to improve survival in unrelated bone marrow (BMT) and HSCT (haematopoietic stem cell transplants) donor transplants, where haplotypes are unknown.
However, it is important to realise that despite the fact that we are targeting BMT as the first commercial target, probes are available for every human chromosome and, therefore, any application for which a researcher may wish to use the technology.
Technology
Essentially, the technology consists of isolating a single metaphase cell from a PHA (phytohemagglutinin) stimulated peripheral blood sample. The metaphase cell solution is exposed to the relevant probe, loaded onto a micro-engineered cartridge, trapped and then lysed.
The labelled chromosomes are then captured separately and sequenced. HT has performed all the steps satisfactorily except for sequencing labelled chromosomes. To date, we have only sequenced unlabelled metaphase chromosomes.
Over the next few months, we intend to establish proof of concept and prepare an investor package to raise additional revenue to enable the construction of the instrument, which will be used up front for most sequencing platforms.
Further uses of technology
Once the first iteration of this technology is launched in 2025, directed at HLA haplotyping for unrelated bone marrow transplantation, what follows, hopefully, will be an avalanche of projects designed to establish haplotypes in various clinical situations, providing a personalised approach to genetic testing These could include:
Establishing CYP gene haplotypes in different populations in order to fully understand individual drug metabolism and resistance. (Waring 2019).
Immunotherapy in different populations involving polymorphic receptors such as CTLA-4 and PD-1 (Breunis, Tan)
Understanding the role of polymorphic genes in multigenic complex diseases such as auto-immune diseases. (Pociot and McDermott)
These are just three examples where haplotyping will benefit personalised medicine. There will be many more both identified and as yet unidentified areas.
We realise that every researcher will have a chromosome they wish to interrogate related to a particular condition. Our original aim was to isolate 46 chromosomes from a cell and identify each one. We quickly realised that this approach would have a high degree of redundancy, and we shifted our focus to identifying chromosomes before separation for the reason given above. There are probes commercially available for each human chromosome.
Collaborating Institutions with HT in this project include:
- University of Adelaide (Adelaide- metaphase chromosome labelling and sequencing)
- Schott Minifab (Melbourne- microengineering of cartridge)
- Planet Innovation (Melbourne- instrument manufacturer)
- Silverpond (Melbourne-AI software for recognition of suitable metaphase cells and identification of labelled chromosomes.)
References
- Kitcharoen K. et al. 2006. Human Immunology; 67(3); pp.238–246.
- Petersdorf E. et al. 2007. PLoS Medicine; 4(1); p. e8.
- Maskalana, M. et al. 2020. Human Immunology 81 pp12-17.
- Waring R. 2020. Xenobiotica 50, (1) pp 9–18.
- Breunis, W B. et al. 2008. J Immunotherapy 31(6), pp 586-590.
- Tan, D. et al. 2018. Cancer Biomarkers 21(2), pp287-297.
- Pociot, F. McDermott, M F. 2002. Genes and Immunity 3, pp 235-249.