Any disease affecting the heart muscle (“myocardium”) is a potential cause of sudden cardiac death. Such diseases include recovery following a heart attack (myocardial infarct “MI”) and diseases, such as Hypertrophic and Dilated Cardiomyopathies (HCM, DCM) together with a host of rarer diseases
Sudden cardiac death (SCD) is caused by a lethal disturbance of the heart’s rhythm – an “arrhythmia”. The majority of arrhythmias are “re-entrant”, meaning that the activation, rather than following an orderly path through the conducting system, follows a self- sustaining circular path, often caused by a scar, which becomes chaotic and turns to ventricular fibrillation (VF) and results in the heart being unable to pump effectively and causes death.
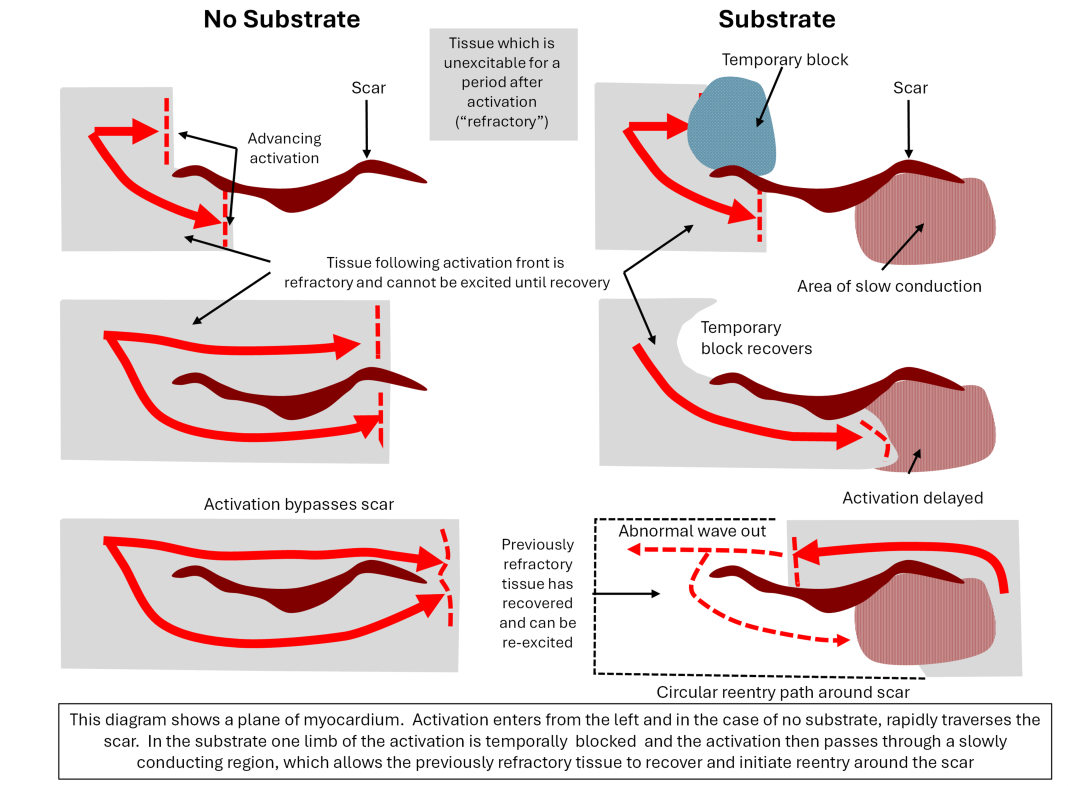
Re-entrant arrhythmias are well understood and have a “substrate”, which are the conditions that allow the arrhythmia to arise. The substrate is not present at rest but arises dynamically, often following an extra beat (“ectopic”) in the myocardium. One component of the substrate is slowed conduction, which allows the myocardium in a re-entrant arrhythmia to recover in front of the advancing wave front. The other condition is a temporary conduction block (See figure below).
SCD prevention
The standard prevention method for SCD is through the implantable cardiac defibrillator (ICD). This device is implanted in the chest wall and has leads in the heart (or tunnelled under the skin outside the heart). It senses an arrhythmia and may deliver a pacing sequence to terminate the arrhythmia, which, if unsuccessful, apply a defibrillating “shock” to return the heart to its normal rhythm.
Prediction of SCD
Rational use of ICDs requires the prediction of patients who are genuinely at risk of SCD as opposed to the majority of patients who are not at risk and do not require implantation. Hitherto, all predictive methods have been based on clinical indicators rather than electrophysiological methods, and these indicators are weakly predictive.
The main indicator for ICD implantation in post-MI patients (Left Ventricular Ejection Fraction) has been shown by several strands of evidence to be random (1), and this has led to the five-fold variation in implantation rates in various European countries. In the U.S., only 5% of the 150,000 ICDs implanted annually ever discharge for an arrhythmia (2) and the therapeutic ratio of ICDs implanted in Europe is under 20%.
In Hypertrophic Cardiomyopathy, an important cause of SCD in young people, various schemes for prediction based on clinical factors have been shown to be distinguishable from random but have minimal predictive capacity. (3)
The ICD is not benign therapy. They are prone to device and lead failure. They are also prone to infection, which carries significant mortality and requires prolonged hospitalisation. Finally, recent studies show that the inappropriate discharge rate (i.e., in a conscious patient for a misdiagnosed arrhythmia) is up to 24% and higher in children.
This leads to extreme distress with psychological sequelae. Thus, there is an imperative to implant high-risk patients and not to implant patients who never develop an arrhythmia.
Electrophysiological prediction of SCD
A technique has been developed to determine if the substrate for arrhythmias is present and to infer the risk of SCD. The principle is to stimulate the cardiac ventricles with a pacing sequence via electrode catheters and record the response at other sites within the heart. The conduction through the myocardium between the stimulating and recording sites can be recorded and repeated for several intervals between successive stimuli.
The recorded signals are processed to emphasise small distinct potentials within them that correspond to different activation pathways between the stimulating and recording electrodes. In diseased tissue, as the interstimulus interval is decreased, the number of potentials increases, and they become delayed, indicating that the activation has traversed disorganised and diseased tissue. In normal tissue, these effects are not present. (4)
This effect (“fractionation”) is very strongly associated with the risk of SCD. It is present in every disease that has been studied (including HCM, DCM, Long QT syndrome and post-MI patients), and techniques have been developed to quantify the effects. A prospective study was performed in HCM, in which asymptomatic patients were examined and followed over five years. Of the 179 patients, 17 suffered an episode of VF, all of whom were assessed as being high-risk. (5)
This has important implications for risk prediction and ICD implantation:
- The method is based on a well-established physiological model of arrhythmogenesis, which is common to most diseases.
- In the case of HCM, it suggests that if 20% of the population were implanted with an ICD, 90-95% of those patients suffering VF would be protected.
- In post-MI patients, the method will hopefully have a high negative predictive accuracy and so be able to eliminate, perhaps, 30-40% of implantations.
It is now clear that attempts to predict arrhythmias based on clinical indicators, which may not be related to the causal chain of events initiating an arrhythmia, do not have adequate predictive capacity to be useful clinically. The research into electrophysiological methods to identify the arrhythmia substrate suggests that a higher predictive capacity is achievable and will benefit patients by reducing ICD implantations, complications and healthcare costs.
References
- European Heart Journal https://doi.org/10.1093/eurheartj/ehae326
- Circulation DOI: https://doi.org/10.1161/CIRCULATIONAHA.123.066984
- EP Europace https://doi.org/10.1093/europace/euad045
- Circulation 1992;86:467-474.
- European Heart Journal https://doi.org/10.1093/eurheartj/ehn111