Tara M. DeSilva from the Department of Neurosciences, Cleveland Clinic, looks at the future of therapeutic strategies for MS, focusing on targeting the central nervous system
Multiple sclerosis (MS) is a demyelinating and neurodegenerative disease initially characterized by white matter lesions in the central nervous system (i.e., brain, spinal cord, and optic nerve) detected by magnetic resonance imaging (MRI). Lesions are caused by the infiltration of immune cells, resulting in loss of the insulation (myelin) around nerve fibers (neuronal axons).
Early in the MS disease course, myelin regenerates; however, over time, sustained demyelination results in axonal injury and permanent disability. (1)
The location of a lesion in the brain, spinal cord, or optic nerve determines the type of deficit a patient with MS will experience. Most patients with MS initially present with a relapsing-remitting disease course. During this phase, manifestations of clinical deficits indicate a relapse followed by a recovery or remittance of symptoms.
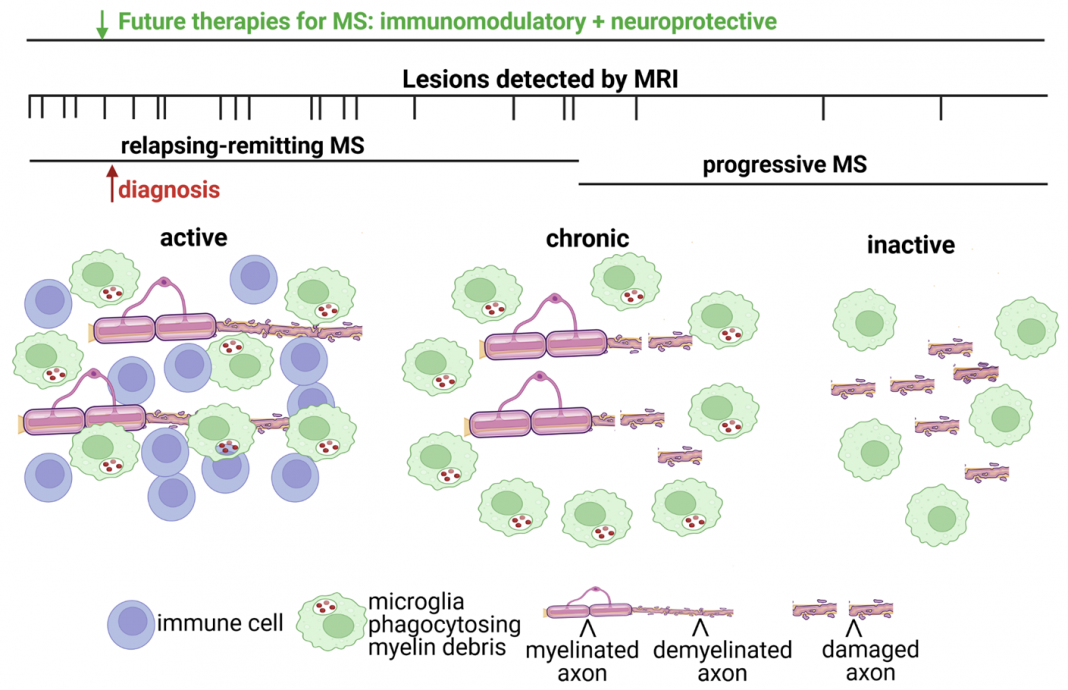
Eventually, MS patients enter into a progressive disease course. During the progressive phase, there is no remittance, and symptoms gradually worsen, leading to permanent disability (Fig. 1).
What treatment strategies for MS already exist?
Current treatment strategies for MS are immunomodulatory by regulating immune cell proliferation, activation, or migration into the CNS. However, these treatments are only effective during the relapsing-remitting phase of MS and do not improve progressive decline.
Understanding the pathological differences between the relapsing- remitting versus the progressive phase of MS is important for developing treatments to prevent long-term progressive decline (Fig. 1).
Why are immunomodulatory therapies not effective in the progressive phase of MS?
The white matter of the CNS is mainly comprised of myelinated axons, while the neuronal cell bodies and synapses are predominantly found in grey matter regions. Aside from oligodendrocytes, cells that form the myelin sheath, the CNS also contains microglia and astrocytes.
Microglia and astrocytes have diverse roles in maintaining neuronal function by removing cellular debris during disease states. Still, they can also become activated and release toxic factors that contribute to neurodegeneration.
There are three types of lesions (i.e., active, chronic, and inactive) found in MS, and differences in their cellular composition may provide important clues as to why immunomodulatory therapies are not effective during the progressive phase of MS (Fig. 1). The cells that distinguish lesion types are peripheral immune cells (T cells, B cells, and macrophages) activated CNS resident glia cells (microglia), and myelin (loss of myelin or demyelination). Active lesions contain CNS-infiltrating immune cells and microglia that are phagocytosing or clearing myelin debris.
Chronic lesions have microglia around the border of demyelinated lesions with damaged axons, while an inactive lesion has complete loss of myelin and extensive axonal damage. Both chronic and inactive lesions lack immune cells, unlike active lesions. Active lesions are predominantly found in relapsing-remitting MS.
Chronic lesions are observed more frequently in progressive MS as well as inactive lesions, which have increased axonal and neuronal degeneration. These lesion types and their association with MS disease states suggest that immune cell infiltration occurs during the relapsing-remitting phase of MS.
However, increased axonal injury correlates with a lack of immune cell infiltration but sustained activation of resident CNS glial cells in the progressive phase.
These pathological findings are consistent with MRI imaging studies demonstrating virtually no new lesions occurring during progressive MS (Fig. 1). Furthermore, recent studies have documented that microglia and astrocytes from chronic lesions in post-mortem MS brains exhibit a neurodegenerative transcriptional profile (2) similar to those found in Alzheimer’s disease.
Taken together, these data support that neurodegeneration in the progressive phase of MS is not driven by peripheral immune cell infiltration into the CNS, which may explain why immunomodulatory therapies are no longer effective.
The central nervous versus the immune system: What to target and when?
Current treatment strategies for MS target the immune system but do not directly target the CNS, reflecting an important unmet need for therapeutic strategies to ameliorate disease progression in MS. It is unknown whether the damage in the CNS is the primary event causing secondary activation of CNS-infiltrating immune cells or aberrant activation of the immune system occurs first, perpetuating their entrance into the CNS.
Nevertheless, immune cell infiltration into the CNS does instigate inflammatory processes that precipitate destruction, warranting immunomodulatory therapies.
Immunomodulatory therapies are effective during the relapsing-remitting phase of MS; however, immune cell infiltration ceases over time, yet neurodegeneration increases with worsening clinical disability.
Studies from the DeSilva laboratory demonstrate that once immune cells enter the CNS, they can activate microglia to release cytotoxic chemicals that damage myelin.(3) One such excitotoxin is glutamate, which is necessary for neurotransmission, but its pathological accumulation can overactive glutamate receptors, causing cell death.
Blocking a source of pathological glutamate (3) or a target for glutamate (AMPA-type glutamate receptors) selectively on myelin (4) not only attenuated demyelination but also preserved the underlying axon.
Therefore, blocking a target for glutamate on myelin provides a dual benefit by preserving myelin as well as the axon with important therapeutic implications for the progressive phase of MS. Furthermore, these studies support that immune cell infiltration activates microglia to perpetuate a neurodegenerative process that destroys myelin and axons.
Myelin and axonal damage occur early in the disease phase with ongoing degeneration even after immune cells are no longer present in the lesion (Fig. 1, chronic and inactive lesions).
Additionally, at the time of MS diagnosis, there is evidence of prior lesion activity by MRI imaging, supporting that the future development of neuroprotective strategies should be administered in combination with immunomodulatory therapies.
References
- Mey, G.M., Mahajan, K.R. & DeSilva, T.M. Neurodegeneration in multiple sclerosis. WIREs Mech Dis, e1583 (2022).
- Absinta, M., et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709-714 (2021).
- Evonuk, K.S., et al. Inhibition of System Xc(-) Transporter Attenuates Autoimmune Inflammatory Demyelination. J Immunol 195, 450-463 (2015).
- Evonuk, K.S., et al. Reduction of AMPA receptor activity on mature oligodendrocytes attenuates loss of myelinated axons in autoimmune neuroinflammation. Sci Adv 6, eaax5936 (2020).

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.