In this article, Alan Herbert, the Founder, and President of InsideOutBio, guides us through targeting the most complex flipon of them all in the fight against cancers
In many treasure hunt games, the final step is usually the most difficult. By that time, you know where the quest is located. However, there still remains a frustrating set of challenges that appear impossible to overcome. As you find potential solutions, you unmask even more devious deterrents to your success. Similar obstacles exist for the treatment of cancers, where we are, more often than not, shooting at a moving target with many defenses.
It is helpful to think back and look at the problem’s origins. Nature started out with nothing but the prebiotic soup built out of thunder and lightning, a toxic mix of chemicals that none of our modern selves would survive. From simple building blocks, Nature tinkered and evolved the complexity of what we see today. While we admire the exquisite specificity of the molecular toolbox that is now encoded in our DNA, finding magic bullets to target those proteins that cause cancers has proven challenging. This failure is not the fault of the drug-maker, but rather reflects that the targets have closely related family members.
The drugs not only hit the target but may also hit these other family members and cause side effects that limit their usefulness. Even when a drug shows the desired efficacy, a single DNA mutation may allow a target to escape control. Other family members may step up to fill the breach, providing another way for a cancer cell to survive. Interestingly, “dirty” drugs that target multiple family members can be more effective in the clinic than the exquisitely designed boutique drugs, albeit with more undesired side effects.
A different molecular toolkit to fight against cancers
We are now learning how to target a different molecular toolkit for the treatment of cancers. The molecules involved enabled Nature to escape from the toxic soup. While much simpler in scope, the pathways they regulate are robust, honed by eons of evolution. Rather than the sequence complexity that marks the modern day, this past history is based on the repetitive sequences that make up the bulk of the human genome.
After all, Nature has to start tinkering somewhere, and these were among the first sequences able to copy themselves. Those repeats that did so created many replicates of themselves, producing long DNA polymers with many sequence repeats. At some point, the repeats were given meaning by the contemporary genetic code in which DNA codons are composed of three letters specifying a particular amino acid with the order of codons specifying the order of amino acids in a protein.
Establishing the code set the stage for repeat sequences to copy themselves more efficiently by encoding proteins, called polymerases, to do the job. Their descendants of these early repeat sequences include the retroelements (called so because their polymerases retro-copy RNA back into the DNA genome) that account for over 50% of the human genome, with only 2.5% of the total sequence encoding the highly specialized proteins in the modern-day molecular toolkit.
These repeat sequences have another feature that is baked into the biology of a cell. They are prone to form alternative DNA conformations. Not only do they form the two-stranded, Watson and Crick right-stranded B-DNA helix, but they also fold in many different ways. The repeat sequences can form the two-stranded left-handed Z-DNA, three (triplexes), and four- stranded DNA structures (quadruplexes).
Each alternative conformation is formed by a unique repeat sequence. The sequences capable of doing this inside the cell are called flipons and are inherited just the same way as genes are transmitted. By changing conformation, flipons act like digital switches that nature tinkers with to program cells. We are just beginning to understand how.
What are Z-flipons and G-flipons?
We know a lot about the biology of Z-flipons. They play roles in the regulation of transcription and immunity. The presence of Z-DNA signals an active gene and enables the reset of transcriptional complexes. The presence of Z-RNA can signal a viral infection or dysfunction of a cell and trigger immune responses that defend against threats that might harm the cell. These pathways are not activated in normal cells. Instead, they are only expressed when cells are stressed and provide a way to target these cells therapeutically.
The biology of G-flipons is more complex. These genetic elements are made from repeats of guanosine (G) that fold into a four-stranded structure called a G-quadruplex (GQ). Many different types of GQ can be formed, depending on the G-repeat sequence. They are much more stable than Z-DNA structures and require unwinding by specialized enzymes, called helicases, to restore the B-DNA state. Indeed, helicase loss of function variants are associated with many genetic diseases characterized by neurological developmental defects and premature aging.
Of course, Nature has found many beneficial ways to exploit the stability of GQ, including the regulation of gene expression, the organization of DNA within the cell nucleus, and the maintenance of a cell state during division. Reflecting their early origins, these G-flipon functions are basic to the biology of the cell. We describe their importance in our recent publications.
Given G-flipons’ impact on a cell, their role in disease has received much attention. In cancer, GQ can promote the productions of cancer-causing proteins, like the famous c-MYC oncogene. Given that GQ comes in different flavors, each with a slightly different set of loops and bulges, they are also potential targets for small molecule therapeutics. However, the huge number of GQ in the repeat genome makes finding the right drug challenging. The risk of unwanted side effects is also high as normal cells also utilize GQ in much the same way as cancer cells.
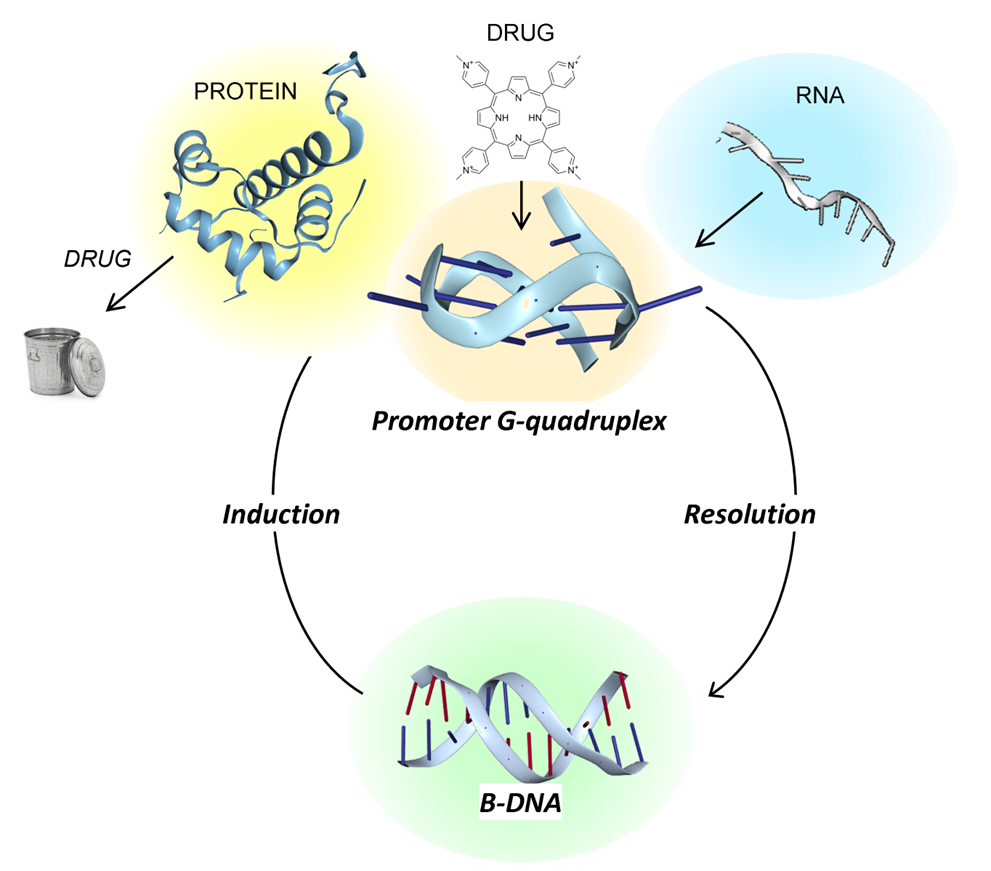
The proteins that control GQ’s formation or resolution
A newer approach hones in on the proteins that control GQ’s formation or resolution (Figure). Many of these proteins are tissue-specific, decreasing the potential side effects of therapeutics that target them. There are certain cancer cells whose survival is absolutely dependent on the presence of one or another of these proteins. One approach is using a molecular glue, such as a PROTAC, that specifically labels such proteins as trash, leading to their disposal. Loss of the proteins then induces the death of the addicted cancer cells.
A less direct approach is to attack the “normal” cells in the stroma that provide logistic support for the tumor. Many of these cells end up in a stressed condition, expressing the Z-DNA-dependent death pathways because of the inflamed environment created by the tumor. Drugs like CBL0137 that induce GQ in stromal cells, such as cancer-associated fibroblasts, also induce Z-DNA formation, triggering the death of these pro-tumor collaborators.
A much different strategy for either enhancing or hindering GQ formation is being explored. The approach depends on small RNAs (or their equivalent) directly targeting GQ by binding to the flipon sequence. These small RNAs can additionally be fused to a small-molecule drug, localizing the effects to a specific GQ that causes disease. Already, some drugs are in clinical trials. Stay tuned as the fight against cancers continues; a lot is happening in this space!
References
- Herbert A. A Compendium of G-Flipon Biological Functions That Have Experimental Validation. International Journal of Molecular Sciences. 2024;25(19). doi: https://doi.org/10.3390/ijms251910299.
- Herbert A. Flipons and the logic of soft-wired genomes. 1st ed. Boca Raton: CRC Press; 2024.