Starting in January 2020, the European REVERT project is now in its final phase. The project blends predictive medicine and AI to enable clinicians to quickly and adequately treat patients
The main objective is to develop a decision support system (DSS) using artificial intelligence (AI) that can assist clinicians in identifying the most suitable combinatorial treatment for individual patients diagnosed with unresectable metastatic colorectal cancer (mCRC).
To achieve its goal, the REVERT project has gathered colorectal cancer data from diverse sources – clinical databases, biobanks’ datasets and real-world data – to assess their potential in identifying the key factors contributing to effective responses against mCRC, as Professor Fiorella Guadagni, the Director of the Biobank BioBIM and Biomarker Discovery and Advanced Technologies (BioDAT) Laboratory at the IRCCS San Raffaele in Rome and the Co-ordinator of the EU-funded REVERT project, explains.
Predictive medicine and AI are central to the REVERT project, featuring a European network of 22 top-performing research and clinical centres located in five European countries as well as long-established biobanks and several small and medium enterprises (SMEs), all contributing through their experience and real-world data. AI models, data management, biomarker discovery, omic sciences, and clinical oncology settings are among the skill sets of all partners, whose focus is on research and development in AI-Health to advance personalised medicine.
Operational synergy in planning activities has been successful for the REVERT Consortium throughout the duration of the project, leading to the development of various AI algorithms that can provide information on disease prognosis and response to currently available drugs.
AI algorithms for a personalised therapeutic intervention
The close collaboration between computer scientists and physicians has been instrumental in all phases of project activities to harness technology’s potential to improve medical treatment. To date, several AI models capable of predicting treatment response against mCRC have been trained. This has been made possible thanks to the availability of large databases on mCRC from a significant number of certified biological banks as well as clinical and research centres that have served for the implementation of an ICT framework for integrating, extracting and harmonising data always in compliance with up-to-date data protection standards.
The consolidation of the REVERT database (RDB) – hosted on an Amazon Web Services bucket – was a prerequisite for the development of a DSS to determine the optimal treatment option for individual patients, which is now being tested in a clinical trial involving patients with unresectable mCRC.
The definition of rational predictive models based on machine learning (https://doi.org/10.3390/ijms22094394) and their implementation (arXiv:2205.06234v2) represented the basis for real-world testing and optimisation of AI predictors, always taking into account that the rationale for a DSS’s suggestion must be understood before using it. This is known as explainable AI.
Analyses were, thus, performed by IT Partners on the RDB using a variety of interpretable machine learning models, where the target variable was the progression-free survival with respect to the date of the first-line treatment.
The best combination of learning results and explainability was finally chosen to deploy the DSS – and a mobile app – to assist clinicians in their decisions.
Notably, although the system is designed to help the oncologist, medical professionals are ultimately responsible for choosing the most suitable treatment for individual patients. Clinicians, indeed, are informed by the system but retain the ability to consider alternative measures while considering the patient’s profile.
In vitro validation of AI models for tailored treatment of mCRC
In addition to the DSS devised for the clinical trial, the ML-based 7-Gene Algorithm, which consists of mutation profiles of seven genes (KRAS, BRAF, ERBB2, MAP2K1, TSC2, TP53, and APC) showed a statistically significant accuracy as a classifier to distinguish between patients who responded to first-line chemotherapy and those who did not (https://doi.org/10.3390/ cancers14082045). Further studies are currently involving next-generation DNA and RNA Sequencing, which are being used to perform drug sensitivity analyses and identify novel potential drugs for improved predictive models for tailored treatment of mCRC.
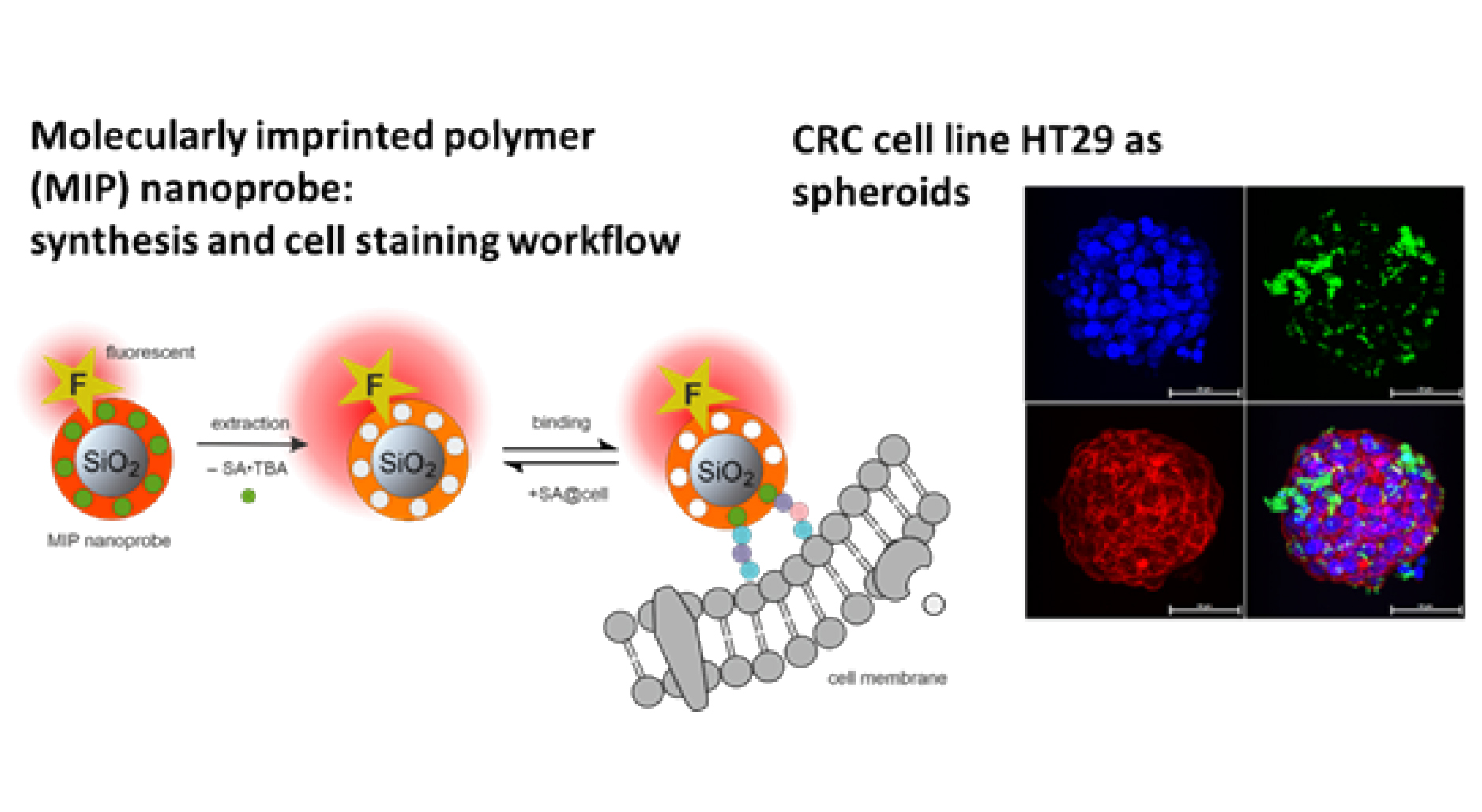
The establishment of organoids from patients’ tumour biopsies and the use of molecularly imprinted polymer (MIP) probes against sialic acid, as used in the REVERT project, provide an interesting setting to test conventional and innovative marker-based drug combinations and compare their effects with the response to treatments administered to the patient from whom the organoid was derived. In addition, organoids are currently being used in experimental studies to validate the algorithm’s ability to accurately predict the response of cells to the most- commonly administered first-line treatment options.
In the end, these studies will provide more chances to investigate the effectiveness of currently available or new combinatorial therapies.
The REVERT Project Clinical Study
Testing and validation of the AI predictive model in the oncology setting in order to improve clinical practice and management of healthcare services for cancer patients is the focus of the REVERT clinical study, a clinical prospective, no-profit, interventional, premarket medical device “early phase” study launched in March 2023 under the co-ordination of the University Hospital ‘Tor Vergata’ of Rome (ClinicalTrials.gov PRS ID: NCT05396807) and the participation of 6 medical oncology units from three different European countries.
The main objective is to aid in the progression of individualised treatment for patients with unresectable mCRC by determining the most effective approach on a case-by-case basis, as explained by the clinical trial coordinator Prof. Mario Roselli, Director of the Medical Oncology Unit at the University Hospital Tor Vergata and Full Professor at the same University.
The DSS – previously trained through retrospective evaluation of the clinical profiles of patients from the RDB, who were defined as ‘responders’ or ‘non-responders’ based on their response to treatment – is currently being applied to new patients who have been enrolled in the clinical study to evaluate its performance in helping investigators choose the best therapeutic option.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


