Curbs on animal testing mean human tissues derived from induced pluripotent stem cells offer a promising platform for discovering novel treatments for cardiac ailments
In the realm of research and development in novel treatments for cardiac ailments, the use of animal models, such as mice, presents inherent limitations due to physiological discrepancies between species, specifically between humans and experimental animals.
For instance, the heart rate in mice can surpass 500 beats per minute, a rate considerably faster than that observed in humans.
These significant physiological disparities impede accurate disease modelling. Moreover, from an animal welfare perspective, it is imperative to curtail the number of animal experiments.
Reflecting this imperative, the FDA recently declared that preclinical animal testing is no longer a prerequisite for human drug trials (Wadman, Science, 2023; 379(6628):127-128). In light of this development, human cardiac tissues derived from stem cells are anticipated to play a pivotal role in the drug discovery processes for cardiac diseases.
Induced pluripotent stem cells (iPSCs)
Cardiac tissues derived from induced pluripotent stem cells (iPSCs) offer a promising platform for cardiac drug research. However, the generation of adult-like myocardial tissues from iPSCs faces multiple challenges.
A critical issue is the immaturity of iPSC-derived cardiomyocytes, which remain in an embryonic state throughout in vitro cultivation, halting the maturation process.
To overcome this, the Yoshida lab developed maturation-reporter iPSC lines that allow for the monitoring of expression levels of TNNI1 (a marker of immature cardiomyocytes) and TNNI3 (a marker of adult-like mature cardiomyocytes), using two fluorescent proteins.
Utilizing this iPSC line, the lab conducted high-throughput screening, identifying several compounds that promote cardiomyocyte maturation.
Notably, an ERRγ agonist was found to induce maturation in iPSC-derived cardiomyocytes, as evidenced by altered gene expression, electrophysiological properties, and mitochondrial functions (Miki et al., Nat Commun, 2021; 12(1):3596).
Patch clamp analyses revealed more mature action potential waveforms in iPSC-derived cardiomyocytes treated with this ERRγ agonist. Notably, ERRγ activation induces T-tubule formation, which is a crucial marker of advanced cardiomyocyte maturation.
Further exploration of this maturation technology confirmed its potential for developing adult-like cardiac tissues. Combined treatment involving mechanical stretching and an ERRγ agonist resulted in the maturation of cardiac myocardial tissues composed of iPSC-derived cardiomyocytes and primary fibroblasts, as evidenced by the upregulation of maturation-related genes, including TNNI3, improved sarcomeric alignment, and increased sarcomere length (Fujiwara et al., Stem Cell Reports, 2023; 18(11): 2108-2122).
Employing these mature cardiac tissues, the team developed disease models of hypertrophic cardiomyopathy characterised by two types of mutations: MYH7 R719Q, which exhibits a severe clinical phenotype, and MYBPC3 G115*, which exhibits a less severe clinical phenotype.
While the mature cardiac tissues derived from MYH7 R719Q mutant iPSCs exhibited various pathological abnormalities related to hypertrophic cardiomyopathy, such as cellular hypertrophy, interstitial fibrosis, diastolic dysfunction, hypercontraction, cardiomyocyte disarray and increased glycolysis, those from iPSCs with the MYBPC3 G115* mutation exhibited only limited pathological findings.
These results suggest that mature iPSC-derived cardiac tissues can effectively recapitulate the cardiac pathology associated with hypertrophic cardiomyopathy and differentiate between severe and less severe clinical phenotypes.
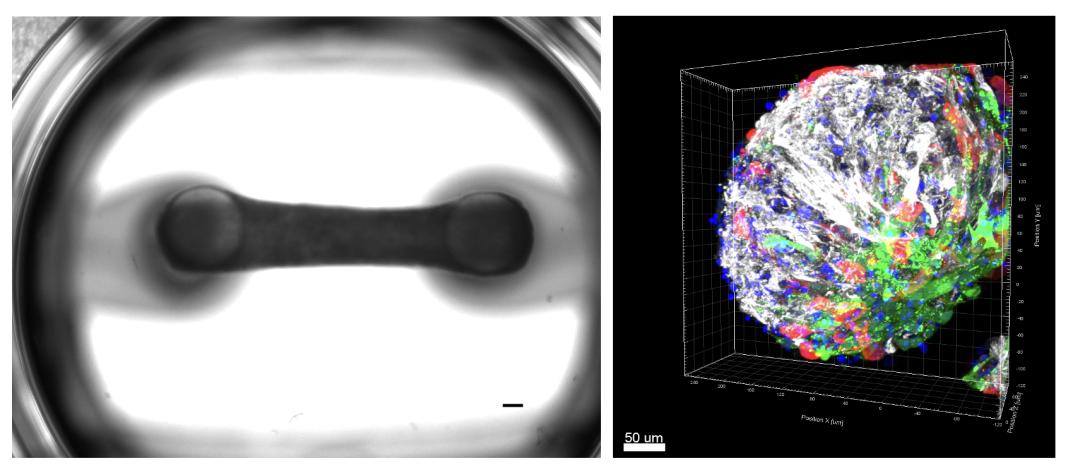
3D cardiac tissues
The research team is currently developing a platform comprising 3D cardiac tissues designed to elucidate the mechanisms of disease progression and to identify potential therapeutic targets and biomarkers.
Furthermore, they are expanding the application of iPSC-derived mature cardiac tissues to facilitate the prediction of disease severity and the investigation of personalized drug responses. These efforts include the generation of 3D cardiac tissues composed of iPSC-derived cardiomyocytes and non-cardiomyocytes, such as cardiac fibroblasts and smooth muscle cells, which more accurately mimic the pathology of a diverse array of cardiovascular diseases.
By harnessing advanced cell and tissue engineering technologies, the team is advancing the development of next-generation 3D cardiac tissues, including cardiac organoids and engineered heart tissues. These innovations are intended for use in drug discovery and toxicology testing, offering potential alternatives to conventional animal models.
iPSC-derived cardiomyocytes represent a promising resource for regenerative therapy. The team has developed a sophisticated method to generate purified iPSC-derived cardiomyocytes using synthetic miRNA-responsive mRNAs, as detailed in recent publications (Miki et al., Cell Stem Cell, 2015; 16(6):699-711; Tsujisaka et al., Stem Cell Reports, 2022; 17(7):1772-1785).
Furthermore, employing bioluminescence imaging, the researchers evaluated the engraftability of iPSC-derived cardiomyocytes at various maturation stages by transplanting them into immunodeficient mouse hearts.
Engraftment capacity
This assessment revealed that cardiomyocytes at a specific maturation stage demonstrated efficient graft formation, highlighting the critical importance of optimising graft cardiomyocytes for successful outcomes (Funakoshi et al., Sci Rep, 2015; 6:19111).
The optimised iPSC-derived cardiomyocytes have shown substantial engraftment capacity when transplanted into the hearts of mice and larger animals, such as monkeys and pigs.
Currently, in collaboration with Orizuru Therapeutics, Inc, the team is preparing for clinical trials targeting cardiac cell therapy for patients with severe heart failure.
Technological advancements
Furthermore, the research team is developing innovative technologies to improve the efficacy and safety of regenerative cell therapy utilising iPSC-derived cardiac cells.
Studies have demonstrated that cardiomyocytes exhibiting active cell cycle activity exhibit a superior capacity for graft formation compared with those with inactive cell cycle activity.
The team has ascertained that AM-80, a retinoic acid receptor agonist, augments the cell cycle activity of iPSC-derived cardiomyocytes, which leads to enhanced graft formation following transplantation into the infarcted hearts of immunodeficient mice (Kasamoto et al., Stem Cell Reports, 2023; 18(8):1672-1685).
Additionally, the transplantation of mature cardiac cells is anticipated to mitigate the risk of arrhythmias by reducing the arrhythmogenic potential of the graft cells.
These technological advancements are expected to significantly bolster the efficacy and safety of cell therapy, potentially paving the way for new treatments that could serve as alternatives to heart transplantation and the implantation of cardiac assist devices.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.