Charles W. Carter, Jr., from the Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, relates molecular recognition used in genetic coding to structures of aminoacyl-tRNA synthetases and their cognate tRNAs
Genetic coding is simultaneously widely understood and little appreciated. Widely understood because Marshall Nierenberg worked out the codon assignments within a decade or so of the double helical structure of DNA by Watson and Crick (1). Little appreciated because a code, as far as we know, is something Nature had never produced – a symbolic mapping of the physical chemistry of the 20 amino acid side chains. The Morse code is a widely appreciated example. Like inheritance, the genetic code is a property of biology.
“Operational RNA code”
I previously described the aminoacyl- tRNA synthetase (AARS)•tRNA cognate pairs that join amino acids to tRNAs containing the symbols necessary to read genes (2). Remarkably, small excerpts of both AARS and tRNA can form similarly functional cognate pairs. The earliest accounts of that notion (3,4) concluded by suggesting that the acceptor stem bases contained elements of the first genetic code, which they called an “operational RNA code”.
Reading a gene means stringing amino acids together according to a blueprint (2,5,6). Natural Selection picks out those blueprints that specify functional proteins. The winners are those sequences that can “fold” into (somewhat) unique 3D structures. That folding is the process that produces function. Here, I’ll summarize how folding serves to pull together what we have learned about the origins of genetic coding.
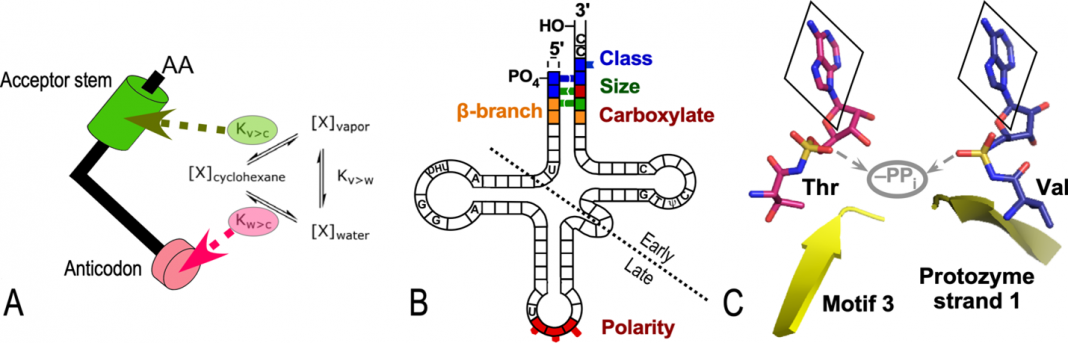
Richard Wolfenden measured two properties of amino acid side chains – size and polarity – that capture their physical chemistry. In the late 1970s, he began recognizing a connection between side chain polarity, folding, and the codon table. The relative affinities of side chains for water explain their distribution between a protein’s surfaces and cores. They also seemed to influence the respective codon middle bases (7) (Fig. 1A).
Considering how AARS recognize their cognate tRNAs
We recently updated those early correlations by considering data on how AARS recognize their cognate tRNAs (8,9).
Folding determines function. So, the next step was determining how the two AARS Classes discriminate between cognate tRNAs (Fig. 1B). The strength of acceptor-stem base pairing determines which direction the 3’ terminus faces. Class I and II AARSs select on
A little later, it became clear that Class I and II AARS active sites also have an inherent bias for selecting either larger (Class I) or smaller (Class II) amino acid side chains (12). The bias arises from a stereochemical difference between the two kinds of active sites. The amino acid α-carboxylate and leaving pyrophosphate group fall on opposite faces of the prochiral α-phosphate of ATP (Fig. 1C). That difference offers more room for larger side chains in Class I active sites.
Thus, the oldest parts of Class I and II AARS of Class I and II AARS could already discriminate between major vs minor groove recognition of tRNA and larger (Class I) and smaller (Class II) side chains, accounting for their ability to discriminate between both RNA and amino acid substrates.
Reflexivity and AARS•tRNA cognate pairs
The ideas summarized in Fig. 1A-C capture a key step in creating reflexivity, the goal outlined in my first essay (5). Reflexivity, in this case, is the self-referential feedback loop that allowed Nature to evolve genetic coding. AARS•tRNA cognate pairs implement the coding table to make sense of gene sequences. Patterns of amino acid size and polarity along the polypeptide chain drive the folding.
Folding is the selective test that drives improvement. Sequences that “make sense” do so by folding reproducibly into long-lived 3D structures that can bind selectively to small, exclusive subsets of amino acids and tRNAs.
Genes and gene products with those limited specificities could then begin to enforce the coding relationships by which they, themselves arose. Using proteins, rather than ribozymes (RNA enzymes), as the assignment catalysts decisively shortens the feedback loop. We have argued that the self-organization of coding via such a tightly closed feedback loop would have been orders of magnitude faster if the assignment catalysts were protein enzymes than had they been ribozymes (13).
AARS urzymes can select subsets of ~four kinds of amino acids from the correct Class about 80% of the time. Thus, four different urzyme•minihelix cognate pairs might have managed a 4-letter coding alphabet. Urzyme•minihelix model systems now permit us to investigate whether or not their minihelix specificities can match that fidelity.
We can understand from Fig. 1 both the detailed assignments in the operational RNA code and their structural basis. Remarkably, the acceptor stem code is much the same as the universal genetic code (11). Nature moved the bases that first signaled AARS binding to the acceptor-stem into a new anticodon stem-loop. That major event may have arisen via doubling and end-to-end joining of minihelix genes (14,15).
References
- J. S. Trupin, et. al., Proc. Natl. Acad. Sci. U.S.A., 1965, 53 807–811.
- C. Carter, OpenAccessGovernment 2023, July, 272-273.
- R. Giegé, Thèse de Doctorat d’Etat, Université Louis Pasteur, 1972.
- P. Schimmel, et. a;.,, Proc. Nat. Acad. Sci. USA, 1993, 90, 8763-8768.
- C. Carter, OpenAccessGovernment 2023, April, 54-55.
- C. W. Carter, Jr. and P. R. Wills, Annual Review of Biochemistry, 2021, 90, 349-373.
- R. Wolfenden, P. M. Cullis and C. C. F. Southgate, Science, 1979, 206, 575-577.
- C. W. Carter, Jr. and R. Wolfenden, RNA Biology, 2016, 13, 145–151.
- C. W. Carter, Jr. and R. Wolfenden, Proc. Nat. Acad. Sci. USA, 2015, 112 7489-7494.
- C. W. Carter, Jr and P. R. Wills, IUBMB Life, 2019, 71, 1088–1098.
- C. W. Carter, Jr and P. R. Wills, Nucleic Acids Research, 2018, 46, 9667–9683.
- C. W. Carter, Jr. and P. R. Wills, BioSystems, 2019, 183, 103979.
- C. W. Carter, Jr and P. R. Wills, Molecular Biology and Evolution, 2018, 35, 269-286.
- W. Möller and G. Janssen, Biochimie, 1990, 72, 361-368.
- M. Di Giulio, J. Theor. Biol., 1992, 159, 199-214.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.