Serge L. Beaucage, Supervisory Research Chemist at the Food and Drug Administration discusses his work with DNA and RNA sequences and the groundbreaking impact this technique could have
Q. To a layman, what is your research primarily for and about?
Synthetic DNA and RNA sequences are currently being used as nucleic-acid-based drugs for the treatment of a plethora of human diseases under antisense and/or RNA interference therapies; the availability of rapid and efficient methods for the chemical synthesis of DNA and RNA sequences has become urgent, particularly in terms of fulfilling increasing demand for therapeutic DNA and RNA sequences. The research projects, currently ongoing in the Beaucage laboratory, are aimed at improving the chemical methods used for the synthesis and purification of these nucleic acid sequences in the context of assembling high-quality nucleic acid-based drugs.
Q. What were the faults with the chemical synthesis process of DNA and RNA sequences?
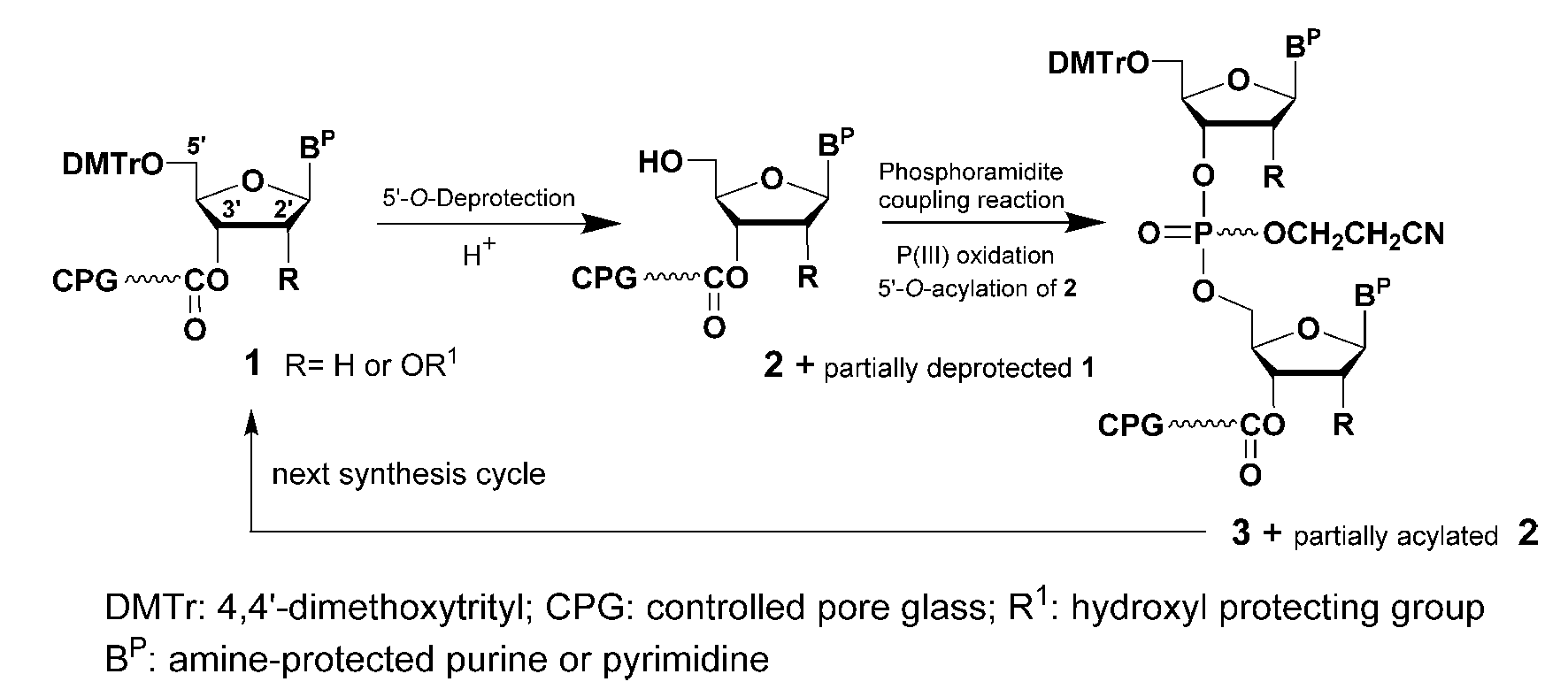
The flaws inherent to the chemical synthesis of DNA and RNA sequences can be better identified through the schemes presented below:
Should 1 be not completely 5’-O- deprotected in the first synthesis cycle, it will then be carried through the next synthesis cycle to produce a dinucleotide while 3 will grow to a tri- nucleotide. Moreover, if unreacted 2 is not completely 5’-O-acylated (capped) in the first synthesis cycle it will also produce a dinucleotide in the next synthesis cycle. Thus, incomplete 5’-O- deprotection of 1 and/or 5’-O-acylation of unreacted 2 will contribute to the formation of shorter than full length DNA or RNA sequences and must be controlled because these shortcomings will be reoccurring after each subsequent synthesis cycle. Optimisation of the 5’-O-deprotection and 5’-O-acylation steps is mandatory for ideal solid-phase synthesis of DNA and RNA sequences. Although such optimisation steps can, to some extent, minimise the formation of sequences shorter than the desired full-length sequence, these contaminants cannot be completely avoided during the manufacture of nucleic acid-based drugs.
Shorter than full-length contaminants, can potentially elicit immune responses and adverse events arising from off- target activities upon administration to patients. Additional manufacturing process-related impurities contaminating synthetic DNA and RNA sequences are created while processing the nucleic acid sequences. Typically, removal of the cyanoethyl phosphate protecting group from the dinucleotide 3, is known to produce acrylonitrile, which can alkylate the pyrimidines of DNA and RNA sequences.
Although the production of these alkylated products had been mitigated, they still pose a threat to the safety and efficacy of DNA- and RNA-based drugs. Process-related impurities can also be generated from synthetic RNA sequences, when the 2’-hydroxyl of each ribonucleoside is protected as an acetal (Beaucage, S. L.: https://doi.org/ 10.1016/B978-0-12-394447-4.10007-0).
Deprotection of an acetal protecting group from synthetic RNA sequences, requires acetal specific chemical treatments, which for the most popular acetal types, will invariably result in the release of formaldehyde (CH2O). Formaldehyde is known to react with the exocyclic amines of the purines and pyrimidines of DNA and RNA to form hydroxymethyl adducts sequences; these sequences were found to be mutagenic in several cell lines, including human cells.
Q. What are the challenges of purifying DNA and RNA sequences?
As mentioned above, process-related impurities including deletion sequences due to failure to either quantitatively prevent the growth of shorter than full-length sequences or to completely remove the 5’-hydroxyl protecting group after each solid-phase synthesis cycle, is a major challenge. Furthermore, the formation of longer than full-length nucleic acid sequences occurs when the activation of phos-phoramidite monomers by a weak acid prompts the premature cleavage of the acid-labile 5’-hydroxyl protecting group of the newly extended nucleic acid sequence; this is a challenge leading to a double DNA or RNA chain extension within the same synthesis cycle. Although the shorter and larger than full-length nucleic acid sequences are produced in small quantities, their physicochemical similarity to the desired nucleic acid sequence makes them very difficult to differentiate through conventional reversed-phase HPLC and anion-exchange HPLC purification methods. Indeed, depending on the nature of individual nucleic acid sequence, more than one purification run may be required to achieve the level of sequence purity required for pharmaceutical applications.
Q. What are the technologies you used to help develop this research?
The Beaucage lab has developed and implemented two technologies aimed at minimising the formation of process-related impurities in synthetic nucleic acid sequences. The first technology has been designed to improve the diffusion of reagents and solvents through the pores of a modified solid support. Thus, a hydroxylated controlled-pore glass support conjugated to three, five or seven hexaethylene glycol spacers was prepared and demonstrated to provide a more efficient and robust process for the synthesis of nucleic acid sequences.
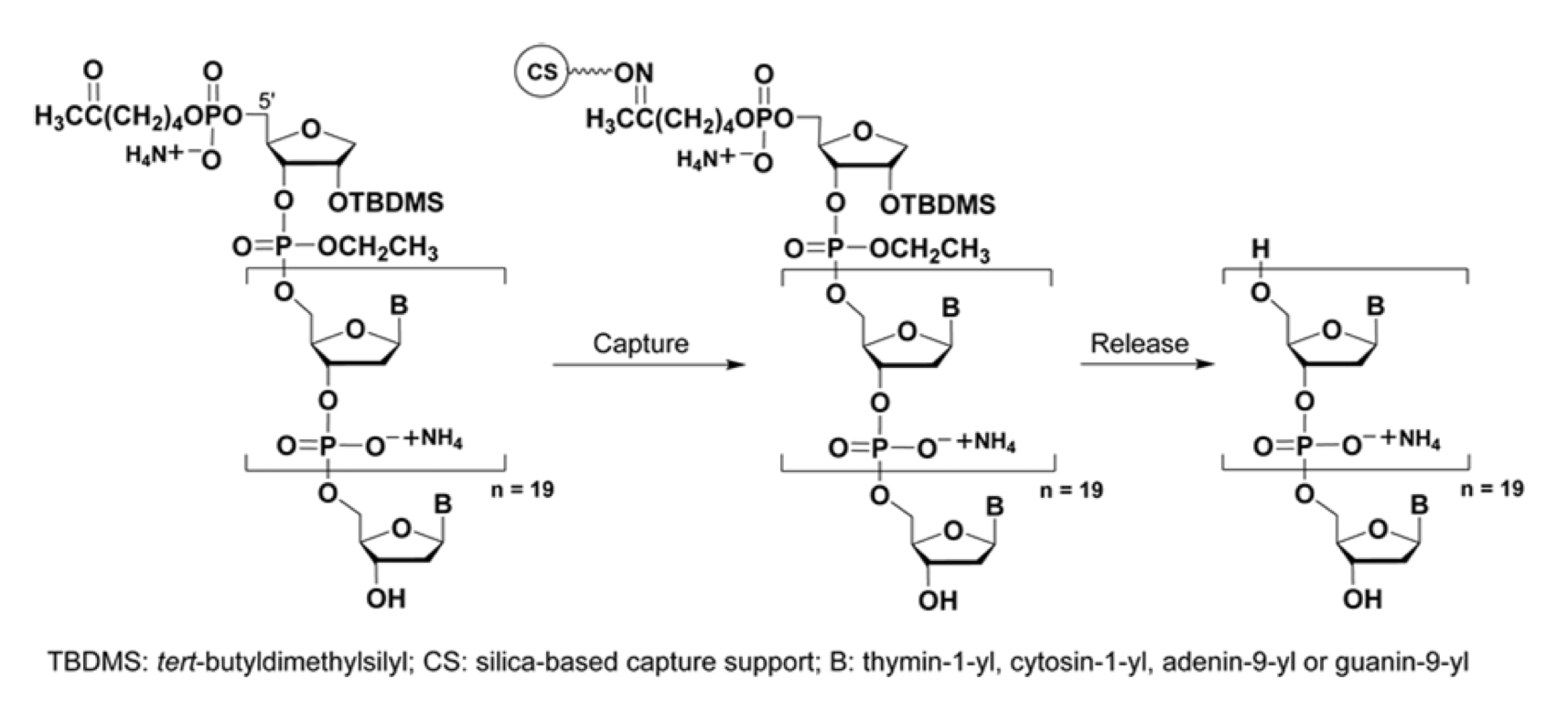
The use of a support conjugated to five hexaethylene glycol spacers led to an up to 42% reduction of process-related impurities contaminating synthetic DNA and RNA sequences, when compared to that obtained from the same DNA/RNA sequences synthesised using a commercial long-chain alkylamine controlled-pore glass support under highly similar conditions (Grajkowski et al.: https://doi.org/10.1016/j.bmc.2020.115779). The second technology entailed the development of a solid-phase capture linker to be incorporated into the last solid-phase coupling cycle of the DNA sequence of interest to yield a unique DNA sequence conjugate. This conjugate is then chemoselectively captured by a solid-phase capture support to permit the unconjugated shorter than full length DNA sequences to be washed off the capture support. The solid-phase-purified DNA sequence is then released from the capture support, through an innovative intramolecular cyclodeesterification reaction and has been isolated in a yield of 94% while displaying an exquisite purity of 97%. (Grajkowski et al.: https://doi.org/10.1016/j.tetlet.2022.154077).
Q. This research can be used for gene therapy and much more, what is the impact this groundbreaking technique could have?
The Beaucage research program provides access to synthetic nucleic acid biomolecules of high purity for the manufacture of antisense, small interfering RNA (siRNA) and microRNA sequences intended for the treatment of human diseases. Given that DNA sequences (>100 nucleotides) are more amenable to manufacturing than RNA sequences, DNA sequences can be chemically or enzymatically ligated to each other to be applied to total enzymatic gene and genome assemblies for genetic engineering manipulations. Large “ligated” DNA sequences can also serve as templates for RNA polymerase to produce equally large RNA sequences to be used for the manufacture of RNA- based vaccines for the treatment of human viral diseases.
Q. Could you talk us through your future plans for DNA and RNA sequences
The future plans of the Beaucage lab are: (i) to complete the research project associated with the implementation of a thermolabile protecting group for the purine and pyrimidine amine functions of deoxyribo- and ribo-nucleosides to be used in the synthesis of DNA and RNA sequences; (ii) to complete the research project associated with the implementation of a thermolabile protecting group for the 2’-hydroxyl of ribonucleosides to be used the synthesis of RNA sequences and; (iii) initiate a project on the development and implementation of a complete thermolytic process for the deprotection and release of synthetic DNA and RNA sequence from the synthesis support. The fully deprotected DNA and RNA sequences can then be solid-phase-purified to provide nucleic acid sequences of sufficient purity for pharmaceutical applications.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.


