Tara M. DeSilva from the Department of Neurosciences, Cleveland Clinic, examines the link between white matter lesions and neurodegeneration
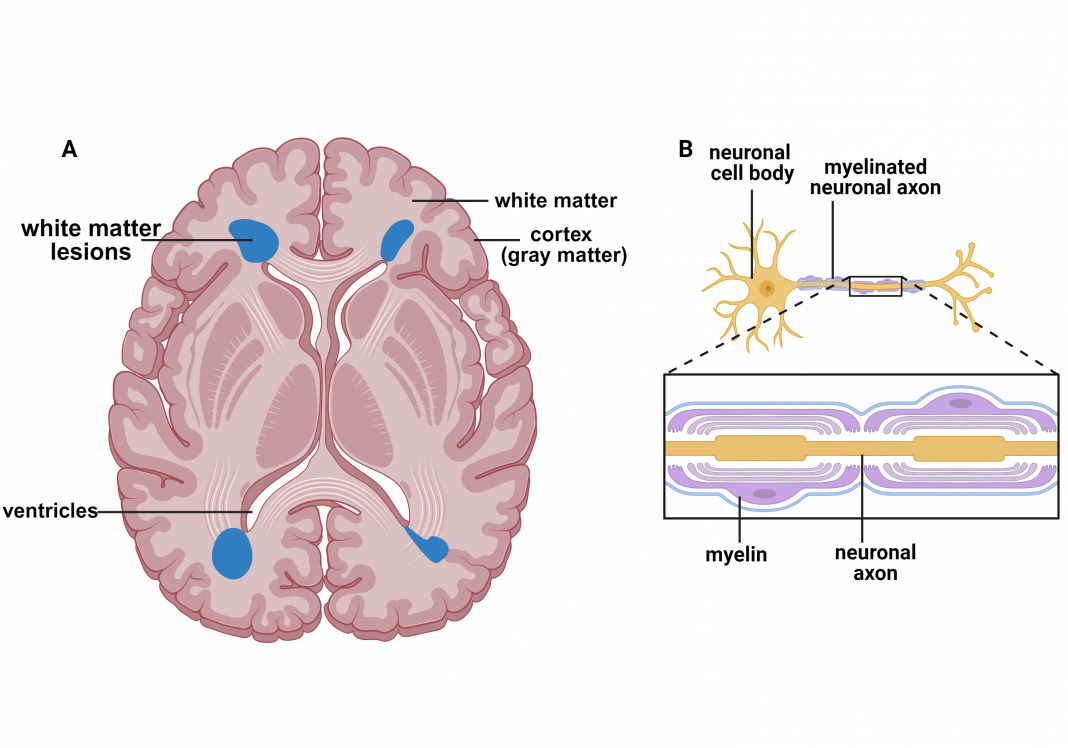
Magnetic resonance imaging (MRI) is a non-invasive technology that provides anatomical assessments of the brain to evaluate pathology. One such pathology is white matter lesions (Fig. 1 A, blue), primarily located around the ventricles. The ventricles are cavities within the brain that produce and transport cerebral spinal fluid surrounding and protecting the brain. The brain’s white matter (Fig. 1 A) houses myelinated neuronal axons (Fig. 1 B).
About myelin
Myelin comprises oligodendrocytes, lipid-rich cells giving it a whitish color, thus the term white matter. Myelin ensheaths neuronal axons (Fig. 1 B) to ensure proper conduction of electrical impulses. The cell bodies of neurons are predominantly located in the cortex, which has a gray color; thus, it is called gray matter.
Neurons transmit information through electrical impulses to communicate with other neurons using specialized connections known as synapses. Myelin facilitates ion channel clustering at nodes of Ranvier along the axon, resulting in saltatory conduction (i.e., jumping of the nerve impulse) that increases the speed of electrical impulses. Damage to myelin impairs the conduction of electrical impulses that allow neurons to transmit information necessary for the normal functioning of the central nervous system, such as cognition, vision, walking, etc.
Multiple sclerosis explored
White matter lesions, referred to as white matter hyperintensities since they appear as bright white spots on an MRI image (Fig. 1 A), are a hallmark of multiple sclerosis (MS). MS is the most common demyelinating disease and is the predominant cause of disability in young adults. The most common initial course, referred to as relapsing–remitting MS, is characterized by acute episodes of clinical disability followed by subsequent recovery. Over time, the improvement after attacks is incomplete, and the relapsing–remitting course evolves into a progressive disabil ity resulting from neurodegeneration.
The underlying pathology driving neurodegeneration in MS is axonal injury found in white matter lesions. Over time, neuronal cell body loss is found in gray matter regions and can be detected by MRI imaging as cortical atrophy. These data suggest that injury to axons causes retrograde loss of neuronal cell bodies.
While studies using models of MS support that a demyelinated axon is more vulnerable to injury than a myelinated axon, it is now known that myelin also provides metabolic support to the axon. Alterations of proteins involved in proper myelin formation not only perturbed myelination, but also resulted in significant axonal damage.
Late-onset axonal degeneration and more
Furthermore, late-onset axonal degeneration without significant myelin pathology was also found in studies when myelin-specific proteins were deleted. Thus, alterations in myelin impair axonal integrity that can lead to permanent axonal loss; however, the signaling mechanisms responsible for this process are not well understood.
One potential pathway involves myelin as a source of energy metabolites for axonal function and survival. Several studies support that deleting proteins involved in lactate production, selectively in myelin, results in axonal demise. Overall, these data support that metabolic coupling between myelin and axons is necessary to maintain axonal health.
Axonal mitochondrial trafficking is another pathway linking myelin to neuronal integrity. Mitochondria are organelles found in most cells necessary to generate energy for cell survival. In axons, mitochondria morphology and trafficking reflect the overall health of the axon. Using different animal models of demyelination, impairments in axonal mitochondrial were observed before evidence of axonal injury was apparent.
One study found that mitochondria in the neuronal cell body were transported to the axon during demyelination as a potential mechanism to preserve axonal function. (1) To test this hypothesis, the investigators enhanced neuronal mitochondrial biogenesis, increasing axonal transport and reducing axonal degeneration during demyelination. (1) These data suggest that demyelination imposes metabolic dysfunction in the underlying axons with grave consequences for permanent axonal demise.
There is also evidence that preventing demyelination during autoimmune demyelination preserves myelinated axons, supporting the idea that myelin is essential for axonal integrity. In studies from the DeSilva laboratory, a protein involved in excitotoxicity was selectively deleted in myelin, which preserved not only myelin but also the underlying axon. (2)
A further understanding of how myelin supports axon integrity could aid in developing neuroprotective therapies for MS patients to ameliorate neurodegeneration.
Alzheimer’s disease/dementia
Interestingly, there is increasing attention to white matter hyperintensities found in the aging brain and their association with dementias, including Alzheimer’s disease (AD). AD is defined by extracellular β-amyloid plaques and accumulation of tau in neurofibrillary tangles, particularly in gray matter regions. While alterations in myelin may occur secondary to neurodegeneration, where neuronal death inevitably leads to degeneration of axons and its ensheathing myelin, clinical studies show evidence of white matter hyperintensities before symptoms of dementia.
Given the vital role of myelin in axonal integrity, these findings suggest that impaired myelin has negative consequences for axonal integrity, thereby enhancing neuronal dysfunction in AD. Aging impacts myelin sheath integrity, in addition to being the greatest risk factor for progression of MS and AD. Whether myelin deficits are primary or secondary outcomes of neurodegeneration, their unequivocal role in axonal integrity has important considerations for multiple sclerosis and other neurodegenerative diseases.
References
- Licht-Mayer, S., et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta neuropathologica 140, 143-167 (2020).
- Evonuk, K.S., et al. Reduction of AMPA receptor activity on mature oligodendrocytes attenuates loss of myelinated axons in autoimmune neuroinflammation. Sci Adv 6, eaax5936 (2020).

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.