Professor Heather Sheardown, C20/20 Ophthalmic Materials Innovation Hub, Department of Chemical Engineering, McMaster University, explores how polymers can be used to treat dry eye disease
Dry eye disease, a multifactorial inflammatory condition that leads to a myriad of symptoms, is becoming a greater societal burden. Among the most common reasons for visits to eye practitioners, moderate to severe dry eye disease is associated with significant pain as well as limitations in performing daily tasks, poor health in general and a higher risk of depression (Craig et al., 2017). The increasing incidence of this condition has led to the development of a host of therapeutic options, either in clinical development or recently clinically available.
Artificial tear substitutes
Aqueous based artificial tear substitutes are commonly used by patients suffering from dry eye disease. A myriad of combinations of viscosity enhancing agents, in particular, synthetic and natural polymers as well as polysaccharides, known for water retention, including various cellulosic polymers, hyaluronic acid, guar and polyethylene glycol have been used in these products. Of particular interest from a drug delivery perspective is the lacrisertTM device. This dissolvable hydroxypropyl cellulose-based insert is typically inserted once per day and dissolves slowly over approximately 12 hours, providing enhancement in the viscosity of the tear film and relief to moderate to severe dry eye patients (Bertens, Gijs, van den Biggelaar, & Nuijts, 2018).
Cyclosporine A, with both immunomodulatory and anti-inflammatory properties, in the form of an ophthalmic emulsion (0.05%), was the first drug approved for the treatment of dry eye disease in the United States (Restasis®, Allergan). A similar emulsion-based formulation, Ikervis® (Santen), has been approved for use in Europe. While widely used, the time to clinically significant improvement as well as the lack of long-term healing with the discontinuation of treatment is seen as detrimental for this drug (Jones et al., 2017). Liftegrast, a small molecule integrin agonist has been approved as a 5% ophthalmic solution (Xiidra®, Shire).
Clinical trials improvement in both signs and symptoms, providing an alternative option for treating dry eye disease (Keating, 2017). Among the most promising of the drugs in clinical trial, lubricin, a lubricating protein normally associated with the synovial fluid, has shown significant efficacy compared with the hyaluronic acid delivery vehicle (Lambiase et al., 2017).
To overcome some of the issues associated with the current formulations, the development of novel formulations for cyclosporine, in particular, has been an active area of research and development. The Cequa® formulation consists of 0.09% cyclosporine in hydrogenated castor oil-based nanomicelle. The formulation, recently approved in the US, is applied twice per day, similar to Restasis® (Sheppard et al., 2020). Clinically, results demonstrated improved dry symptoms scores as well as an improvement in the production of tears. Novaliq is developing a clear non-aqueous semi-fluorinated alkane-based Cyclosporine A solution (Wirta et al., 2019).
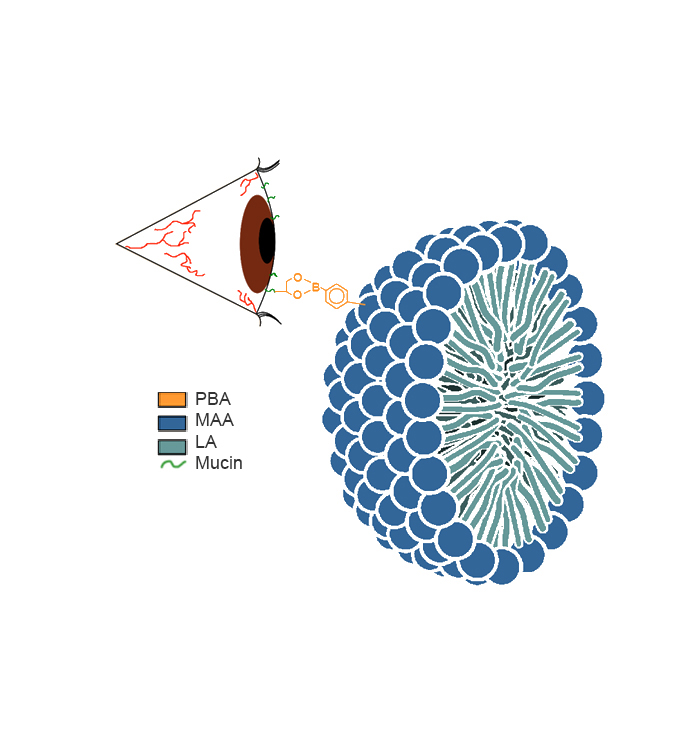
This solution is reported to have greater bioavailability compared with the emulsion-based formulations and showed consistently improved patient scores relative to the vehicle or Restasis®. More recently, two mucoadhesive formulations that came out of work in the 20/20 NSERC Ophthalmic Research Network have been reported (Liu et al., 2015; Liu et al., 2016; Prosperi-Porta, Kedzior, Muirhead, & Sheardown, 2016) (See Figure). The 20/20 OptimEyes formulation is in preclinical validation and shows good results in a dry eye disease model with cornea levels approximately four times higher than Restasis® on the same dosing schedule.
The potential for prolonged delivery to enhance the efficacy of well-established drugs such as cyclosporine is relatively untapped, but with a great deal of potential. As noted by Nichols et al. (Nichols, Evans, & Karpecki), and as evidenced by the use of these novel polymer-based formulations for cyclosporine, the importance of the vehicle for the delivery of these drugs cannot be underscored and there is a real need for new vehicles which provide better delivery of drugs and better bioavailability. Even with the introduction of new drugs to treat dry eye disease, better formulations will be critical for improving drug bioavailability as well as patient comfort.
References
Bertens, C. J. F., Gijs, M., van den Biggelaar, F., & Nuijts, R. (2018). Topical drug delivery devices: A review. Experimental Eye Research, 168, 149-160. doi:10.1016/j.exer.2018.01.010
Craig, J. P., Nelson, J. D., Azar, D. T., Belmonte, C., Bron, A. J., Chauhan, S. K., . . . Sullivan, D. A. (2017). TFOS DEWS II Report Executive Summary. Ocular Surface, 15(4), 802-812. doi:10.1016/j.jtos.2017.08.003
Jones, L., Downie, L. E., Korb, D., Benitez-Del-Castillo, J. M., Dana, R., Deng, S. X., . . . Craig, J. P. (2017). TFOS DEWS II Management and Therapy Report. Ocular Surface, 15(3), 575-628. doi:10.1016/j.jtos.2017.05.006
Keating, G. M. (2017). Lifitegrast Ophthalmic Solution 5%: A Review in Dry Eye Disease. Drugs, 77(2), 201-208. doi:10.1007/s40265-016-0681-1
Lambiase, A., Sullivan, B. D., Schmidt, T. A., Sullivan, D. A., Jay, G. D., Truitt, E. R., . . . Mantelli, F. (2017). A Two-Week, Randomized, Double-masked Study to Evaluate Safety and Efficacy of Lubricin (150 mu g/mL) Eye Drops Versus Sodium Hyaluronate (HA) 0.18% Eye Drops (Vismed (R)) in Patients with Moderate Dry Eye Disease. Ocular Surface, 15(1), 77-87. doi:10.1016/j.jtos.2016.08.004
Liu, S. Y., Chang, C. N., Verma, M. S., Hileeto, D., Muntz, A., Stahl, U., . . . Gu, F. X. (2015). Phenylboronic acid modified mucoadhesive nanoparticle drug carriers facilitate weekly treatment of experimentallyinduced dry eye syndrome. Nano Research, 8(2), 621-635. doi:10.1007/s12274-014-0547-3
Liu, S. Y., Dozois, M. D., Chang, C. N., Ahmad, A., Ng, D. L. T., Hileeto, D., . . . Gu, F. X. (2016). Prolonged Ocular Retention of Mucoadhesive Nanoparticle Eye Drop Formulation Enables Treatment of Eye Diseases Using Significantly Reduced Dosage. Molecular Pharmaceutics, 13(9), 2897-2905. doi:10.1021/acs.molpharmaceut.6b00445
Nichols, K. K., Evans, D. G., & Karpecki, P. M. A Comprehensive Review of the Clinical Trials Conducted for Dry Eye Disease and the Impact of the Vehicle Comparators in These Trials. Current Eye Research. doi:10.1080/02713683.2020.1836226
Prosperi-Porta, G., Kedzior, S., Muirhead, B., & Sheardown, H. (2016). Phenylboronic-Acid-Based Polymeric Micelles for Mucoadhesive Anterior Segment Ocular Drug Delivery. Biomacromolecules, 17(4), 1449-1457. doi:10.1021/acs.biomac.6b00054
Sheppard, J., Kannarr, S., Luchs, J., Malhotra, R., Justice, A., Ogundele, A., . . . Bacharach, J. (2020). Efficacy and Safety of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, for the Treatment of Keratoconjunctivitis Sicca: Pooled Analysis of a Phase 2b/3 and Phase 3 Study. Eye & Contact Lens-Science and Clinical Practice, 46, S14-S19. doi:10.1097/icl.0000000000000636
Wirta, D. L., Torkildsen, G. L., Moreira, H. R., Lonsdale, J. D., Ciolino, J. B., Jentsch, G., . . . Krosser, S. (2019). A Clinical Phase II Study to Assess Efficacy, Safety, and Tolerability of Waterfree Cyclosporine Formulation for Treatment of Dry Eye Disease. Ophthalmology, 126(6), 792-800. doi:10.1016/j.ophtha.2019.01.024
*Please note: this is a commercial profile