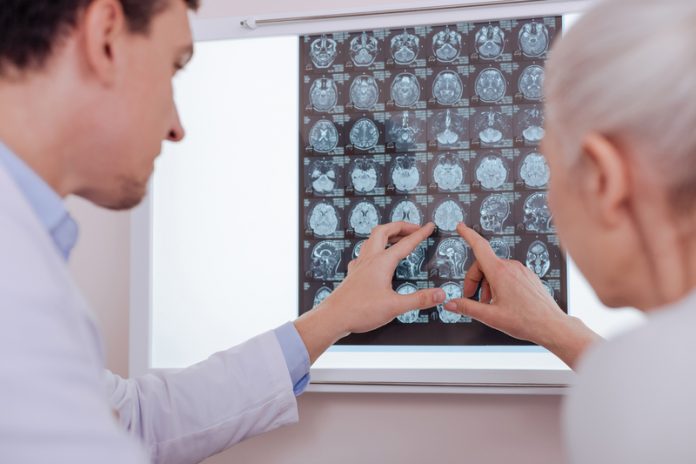
With the Government set to invest an additional £20 million into the research, diagnosis and development of treatments for brain tumours, Dr Ali Hansford elaborates on the need to talk more about how we are going to find the next blockbuster treatments for these devastating diseases
Nearly 11,500 people are diagnosed with a brain tumour every year in the UK with fewer than 15% surviving beyond 10 years[1]. This week’s announcement from the from the Department of Health and Social Care – following the death of Dame Tessa Jowell – that they would be doubling investment for brain cancer research to £40 million is a welcome commitment to helping achieve a goal our industry shares: finding innovative new treatments and cures for these diseases.
The science is advancing in laboratories here in the UK and around the world, funded and supported by charities, universities and the pharmaceutical industry, collectively we are working to fight back against this terrible disease.
Among the 7,000 medicines currently being developed by the global pharmaceutical industry, there are 58 medicines in the pipeline for brain tumours, including gliomas[2]. Companies are actively working to find better ways to speed up medicines development to get treatments to patients sooner.
In her speech to the House of Lords in January, Dame Tessa Jowell talked candidly about her glioblastoma diagnosis and called for greater collaboration in the fight against cancer. She also talked about the speeding up of drug trials by testing more than one at a time, saying: “I am not afraid, but I am fearful that this new and important approach may be put into the ‘too difficult’ box.”
The type of clinical trials Tessa Jowell talked about have many different names: adaptive randomisation, drop-the-loser, adaptive dose-finding, adaptive seamless and the list goes on.
The one thing they all have in common is flexibility. In trials like this – that we call adaptive design clinical trials – researchers can see how patients are responding to treatments and then change or stop parts of the trial in real time.
When used appropriately, trials like this may improve efficiency, reduce cost, maximise information gained and minimise risk to the patients and sponsors. Ultimately, drug development can be accelerated so that the right treatments can be delivered rapidly to the right patients. The UK is seen as a pioneer of innovative clinical trials and this involves collaboration between academia, the NHS, industry and medical research charities – we must ensure we keep it that way in the future.
The issue is that these clinical trial types are not easy to design, plan or execute. An adaptive design will not rescue a poorly planned trial or ineffective treatment.
We need to make sure the regulatory authorities in the UK are not seen as a barrier to innovation; the MHRA and HRA are open to discussion and we need to encourage researchers and pharmaceutical companies to start conversations with them early in the process of planning an innovative clinical trial.
We think that adaptive design clinical trials could be the solution to speeding up the research and development of not only brain tumour treatments, but for all sorts of diseases. Research into small or rare patient populations could really benefit from these trials since they help us quickly rule out the drugs or drug combinations that aren’t working and give more patients the option to contribute to research and clinical trials.
We’re not alone. In February, the Department of Health and Social Care published their brain tumour research report which stated that, because brain tumours are one of the areas that have small patient populations, we need to think differently about how we conduct clinical trials and incorporate innovative trial designs.
The report provided practical recommendations for how we can work collaboratively to make quicker progress in this area. The next steps are to build on the UK’s existing strengths, ensure we have access to researchers with the right skills, and make sure that the right infrastructure is in place for us to make really make progress in this area.
Alongside their funding announcement, we welcome the Government’s commitment this week to accelerate the use of adaptive design trials. When used appropriately, drug development can be accelerated so that the right treatments can be delivered rapidly to the right patients – and that’s where the real benefit lies.
As we look to the future of cutting-edge research and development for blockbuster treatments, we know we need to make the case for innovative clinical trial design, talk more about the amazing science our researchers, companies and NHS are pioneering and encourage them to have open conversations with the UK regulators to ensure that innovative clinical research is safe and effective.
Together, we won’t rest until devastating brain tumours are a thing of the past.
References
[1] Cancer Statistics: Brain, other CNS and intracranial tumours statistics (Cancer Research UK, Accessed: 2018)
[2] Medicines in development for cancer: More than 800 medicines and vaccines in clinical testing for cancer offer new hope to patients (PhRMA, 2015)
Dr Ali Hansford
Head of Science Policy
ABPI