Using cross-disciplinary technology, Dr Kunio Matsumoto, PhD, Professor at Kanazawa University in Japan is extending research on growth factor toward synthetic biologics for regeneration-based medicine and cancer theranostics
Growth of normal cells in our body fundamentally depends on extracellular bioactive proteins called “growth factors”. A variety of growth factors play indispensable roles in the development and regeneration of tissues. Tissues and organs in our body have an ability to, more or less, regenerate following injury and diseases, in which growth factors support the intrinsic ability to regenerate. Growth factors not only govern cell growth but also powerfully promote cell survival, cell migration, and tissue formation at extremely low concentrations, and therefore have great potential as biological drugs for therapeutic use. Several kinds of recombinant growth factors are used for treatment of patients.
Hepatocyte growth factor
Hepatocyte growth factor (HGF) was discovered as a growth-promoting factor for hepatocytes. HGF affects the cell by binding to its cell membrane-spanning receptor, MET. Like a key, HGF binds to MET as a keyhole in highly specific manner, which leads to activation of MET receptor. The MET receptor is expressed in a variety of cells such as hepatocytes, neurons, gastrointestinal cell, skin cells, etc. Dr Kunio Matsumoto, Professor, Nano Life Science Institute and Cancer Research Institute at Kanazawa University, Japan, has spent much of his career investigating HGF. He also started a venture to facilitate clinical development of recombinant HGF for therapeutic purpose. Double-blind, randomised and placebo-controlled clinical trials have been progressed for treatment of patients with spinal cord injury (phase-I/II) and amyotrophic lateral sclerosis (ALS) (phase-II).
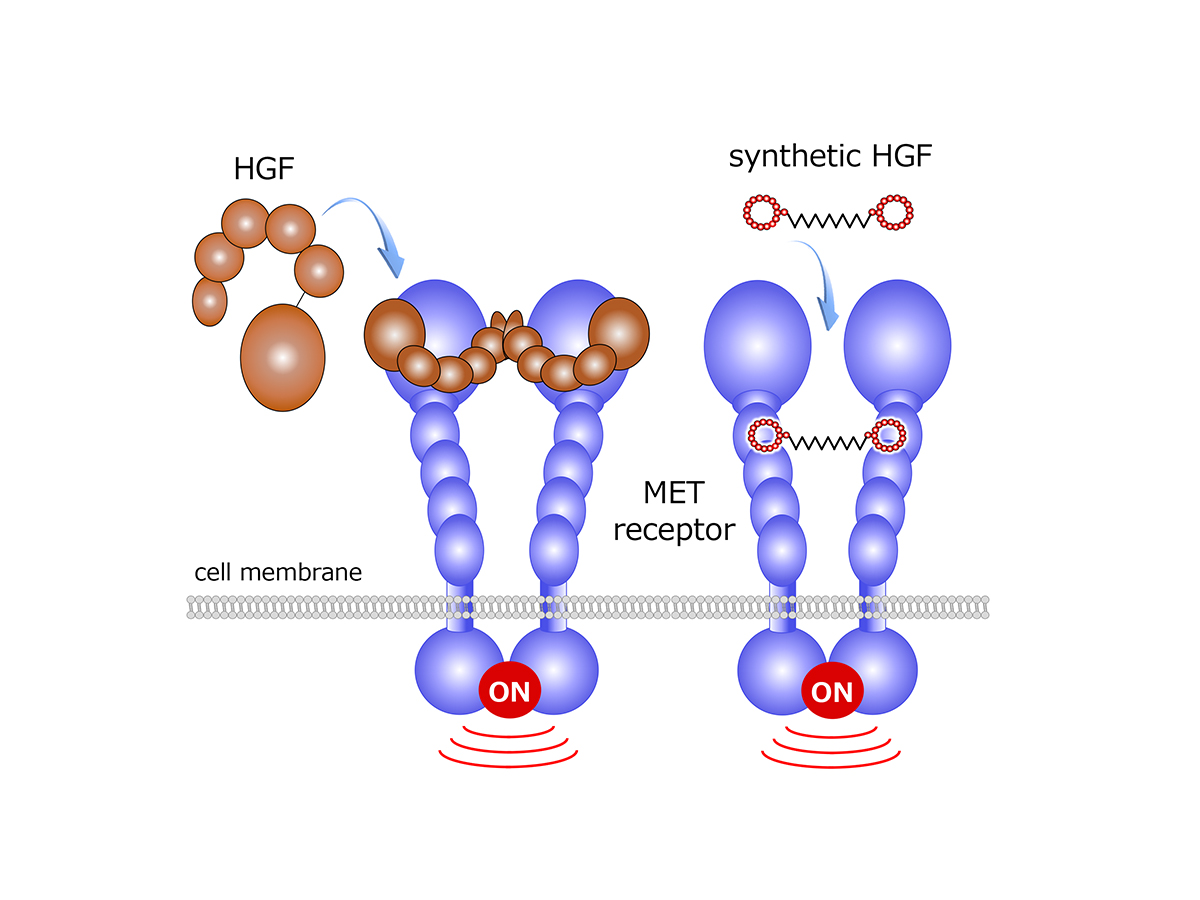
Macrocyclic peptides
Kunio has extended his research through cross-disciplinary collaboration with Dr Hiroaki Suga, Professor, Graduate School of Science at the University of Tokyo. Hiroaki originally established an innovative technology “RaPID (Random nonstandard Peptide Integrated Discovery)” system which enables to discover cyclic peptides against pharmaceutical targets.
Peptides and proteins are both composed of amino acids, while peptides are much smaller than proteins. In RaPID system, peptide library with diversity over 1013 is screened as binders to a target protein and small peptides with circular structure composed of 10–15 amino acids are finally obtained. Kunio and Hiroaki co-worked to discover the artificial HGF composed of cyclic peptides capable of activating the MET receptor (see figure 1). The synthetic HGF shows biological activities equivalent to native HGF, indicating the creation of almost perfect synthetic HGF. Recombinant growth factor drugs can be replaced by synthetic growth factors in future, which enables a much lower cost-performance.
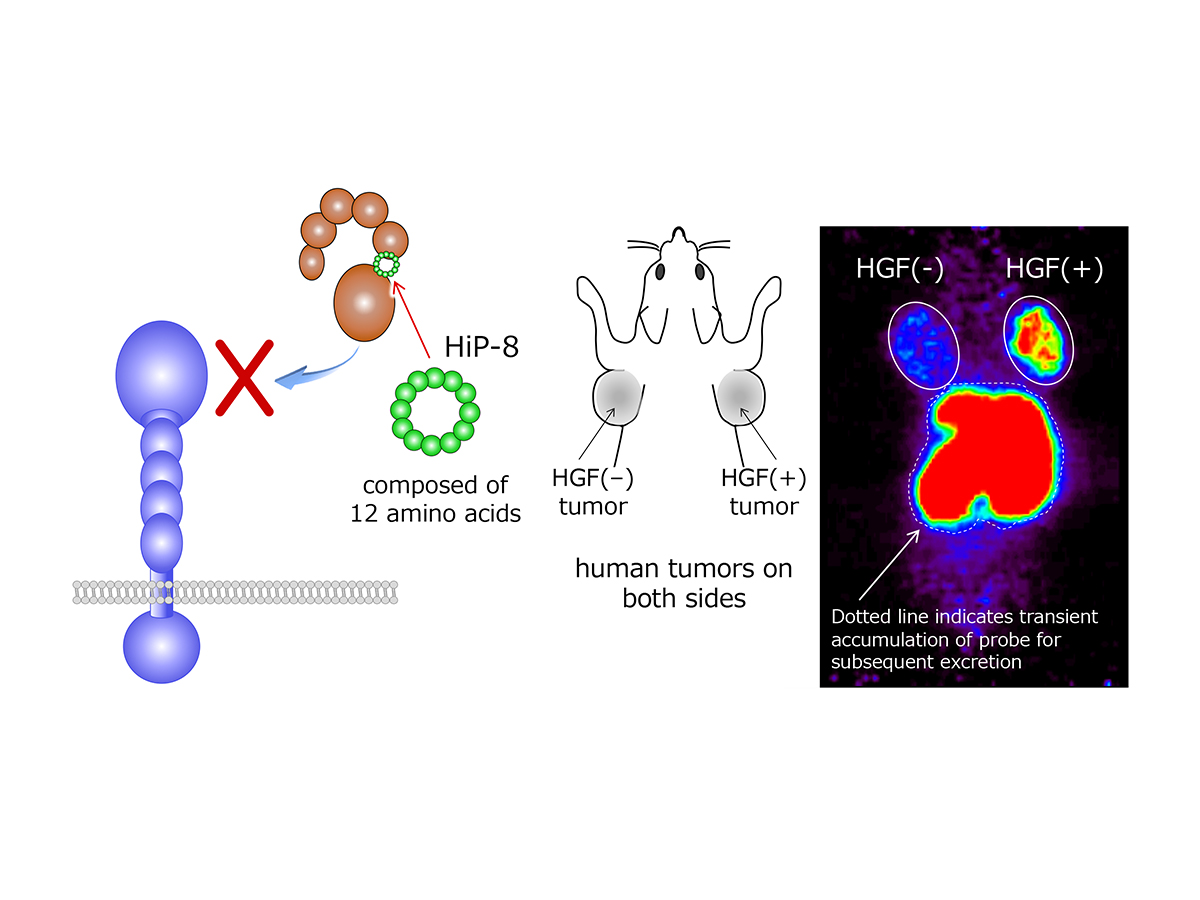
Targeting HGF for cancer diagnosis and therapeutics
Biological pathways often have an important role in more than one process. Processes that are essential for normal physiology are hijacked by diseases such as cancer. The same pathways that are critically important for recovery from tissue injury or other diseases can assist tumour growth. HGF promotes the regeneration and reconstruction of damaged tissues, however, cancer cells commandeer the HGF-MET pathway to promote their own spread. HGF-MET pathway activation leads to an increase in the cell’s ability to grow and move in its environment, i.e., spreading. Likewise, HGF-MET pathway activation leads to an increase in the cell’s ability to survive even in a stressed condition. Thus, HGF causes cancer cells to become both more invasive/metastatic and more resistant to anticancer drugs.
Kunio commented: “We have a two-pronged approach for the clinical application of HGF. One is the use of HGF and synthetic HGF as regeneration-based therapy for treatment of diseases to facilitate regeneration of tissues. The other is use of an inhibitor and/or a probing molecule of HGF for cancer detection and inhibition.”
Using RaPID screening system, the collaborating group by Kunio and Hiroaki recently discovered an inhibitory cyclic peptide, named HGF-inhibitory peptide-8 (HiP-8) (see figure 2). HiP-8 composed of 12 amino acids binds to HGF with outstanding selectivity and affinity. Once HiP-8 binds to HGF, HGF cannot bind to the MET receptor. Highly selective ability of HiP-8 to bind HGF was tested to check its potential as a molecular probe for positron-emission tomography (PET) imaging diagnosis in preclinical model. HiP-8 was excellently accumulated in cancer tissue wherein HGF levels were higher, indicating non-invasive visualization of HGF-positive cancer.
In 2017, a new project for the Nano Life Science Institute (NanoLSI) at Kanazawa University was selected as a project in the World Premier International Research Center Initiative (WPI program) by the Japanese government. Kunio is also working in this new project. The goal of WPI-NanoLSI is to bridge expertise in bioscience and scanning probe microscopy such as high-speed atomic force microscopy (AFM), world-leading technologies developed at Kanazawa University. By using high-speed AFM, Kunio’s team succeeded to observe a movie for dynamic change in HGF before and after HiP-8 binding. The molecular shape of HGF was dynamically changing, but it was suppressed by HiP-8. A small molecule inhibits dynamic movement of a large molecule. As Kunio explains, “The most fantastic characteristic point is that of dynamic and real-time movement of biomolecules can be observed by high-speed AFM.”
Please note: This is a commercial profile











