Jay Sibbitts, Shu Jia, Obdulia Covarrubias-Zambrano, Stefan H. Bossmann and Christopher T Culbertson from Kansas State University’s Department of Chemistry walk us through what we need to know about microfluidic devices for detecting pain
To overcome the current opioid crisis in the U.S., there is a critical need for physicians to be able to quantify pain with biomarkers and assess opioids and metabolite levels at the point-of-care with the goal to better manage pain and avoid overprescribing. The development of novel rapid non-invasive sensing platforms that can be used for the detection and quantification of chemical markers of opioid use that may be present in body fluid, such as urine and blood, as well as biomarkers of pain that can be detected in body fluid.
The Culbertson and Bossmann groups are working on: 1) developing point-of-care (POC) devices that can be used in clinical settings to guide healthcare providers in profiling and quantifying robust molecular biomarkers of pain to determine the optimum dose of a drug that may be prescribed to manage pain symptoms in chronic pain patients, and 2) developing sensors that can help physicians identify patients whose pain management can benefit from the use of pain killers.
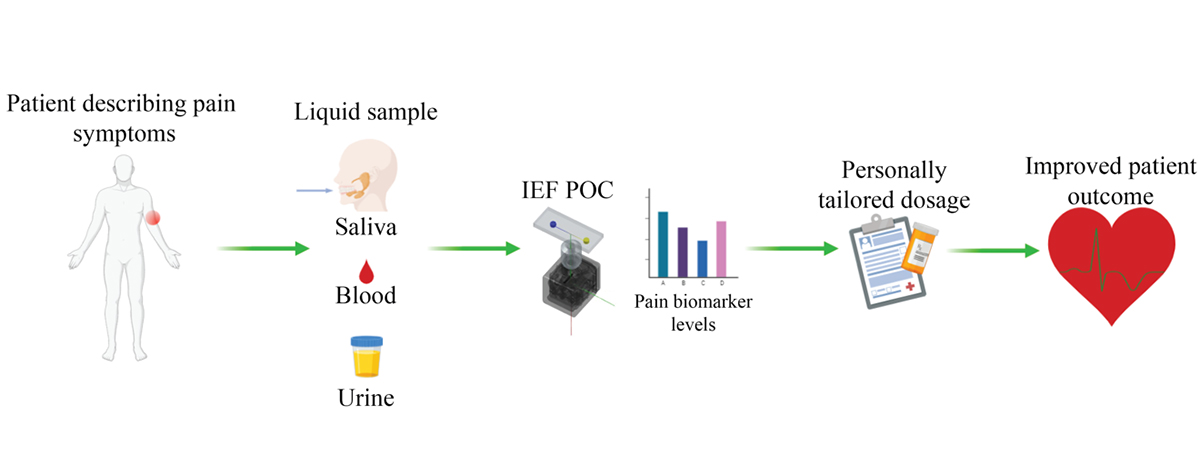
Microfluidic devices
Microfluidic devices are capable of manipulation of minimal sizes of fluids equivalent to the size of a single cell. Due to the compact instrument sizes and widely available automated control, microfluidic devices are often adopted in point of care medical tests, which allow medical workers to take samples and perform diagnosis with minimal costs and labour. A POC device requires components for electronic control, fluid transport and detection to be compact and preferably inexpensive. Our group focus is on the development of POC devices for enzyme activity assays and single-cell detection.
Activities of critical inflammatory responsive enzymes, such as Matrix Metalloproteinases (MMPs) can aid the diagnosis of a breast infection, neuropathic pain and cancer metastasis, but due to the complicated sample preparation of traditional detection methods, very few medical facilities provide an activity-based diagnosis. To address this, the Culbertson group has collaborated with the Bossmann group to provide simple sample treatments that can be integrated into POC devices. Enzyme substrates are loaded onto magnetic nanoparticles that can be retrieved from a complicated sample matrix, such as blood. Samples containing enzymes of interest can be directly injected into the microchip and react with substrates without any sample pre-treatments.
In addition, a custom-built magnetic ‘tweezer’ is used to manipulate the movements of nanoparticles on-chip, such as mixing with samples and retrieval of nanoparticles. Compared to traditional wet chemistry assays, this method minimises sample processing effort as well as errors generated in the process.
Sample throughput
Aside from sample processing, increased sample throughput is another important advantage offered by microfluidic POC devices. To improve the sample throughput, multiple substrates can be labelled with a fluorescent dye and loaded onto magnetic nanoparticles for simultaneous measurement. After incubating the nanoparticles with enzyme-containing samples, reacted and unreacted substrates are transferred to the separation channel for isoelectric focusing (IEF). Upon application of a DC electric field, substrates are separated and focused into small bands based on their isoelectric points. For detection, a laser-induced fluorescence (LIF) setup is aimed at the IEF channel initially. A programmable linear movement stage carries the microchip back and forth to allow LIF detection to scan fluorescence signals of reacted and unreacted substrates at difference isoelectric points. (Figure 2). Depending on the design of nanoparticles, this setup can offer three to five times the throughput of traditional methods and require minimal human input. The increased efficiency and lower cost can make enzyme activity assays more accessible in clinical environments.
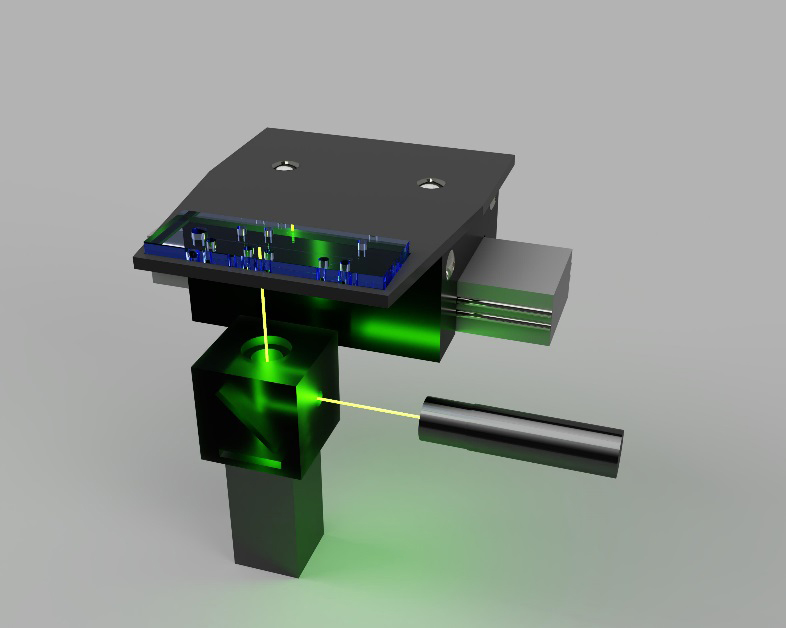
Control electronics
Beyond the work toward robust scanning IEF capabilities, our groups have also been working to develop microfluidic devices that use compact inexpensive control electronics, integrated fluid handling and novel optical fibre detection strategies. The recent increase in prevalence and availability of low-cost electronics, such as single-board computers and microcontrollers, has resulted in their growing widespread use in the research laboratory. Full-sized computers that generally cost hundreds of dollars can be replaced with a credit-card-sized circuit board costing as little as $30.
Additionally, open-source microcontroller boards smaller than a stick of chewing gum can be used to control moving components, actuate valves, or even regulate the device temperature to 37°C for enzymatic reactions. The scanning IEF device is currently controlled by these miniaturised electronic components decreasing its overall footprint and cost.
In addition to control electronics, integrated fluid control and optical fibre detection represent two key steps toward developing a POC device. The on-board pumps we have developed have been used in an automated single-cell analysis device for the transport of cells within a microchannel.1-4 These pumps can be feasibly integrated into the IEF POC device for automated fluid handling in multistep sample processing. After sample processing, optical detection is the next issue to be addressed in the POC device.
Recently our work has been centred around the use of integrated optical fibre detection in microfluidic devices.1, 3-4 The devices used a thin optical fibre to form an optical bridge that allows for simultaneous detection at two points that can be spaced hundreds of microns or even tens of millimetres apart without any additional optics. This detection scheme is possible because of the flexibility of the optical fibre and the relatively small diameter of the fibre face (125 μm). The optical fibre’s small footprint and ability to transmit and receive light even while being bent makes it an attractive alternative to bulkier optics for scanning detection in the POC device.
References
- Sibbitts, J.; Sadeghi, J.; Culbertson, C. T., Microelectrophoretic single-cell measurements with microfluidic devices. Methods Enzymol 2019, 628, 223-241.
- Patabadige, D. E.; Mickleburgh, T.; Ferris, L.; Brummer, G.; Culbertson, A. H.; Culbertson, C. T., High-throughput microfluidic device for single cell analysis using multiple integrated soft lithographic pumps. Electrophoresis 2016, 37 (10), 1337-44.
- Patabadige, D. E.; Sadeghi, J.; Kalubowilage, M.; Bossmann, S. H.; Culbertson, A. H.; Latifi, H.; Culbertson, C. T., Integrating Optical Fiber Bridges in Microfluidic Devices to Create Multiple Excitation/Detection Points for Single Cell Analysis. Anal Chem 2016, 88 (20), 9920-9925.
- Sadeghi, J.; Patabadige, D. E.; Culbertson, A. H.; Latifi, H.; Culbertson, C. T., Out-of-plane integration of a multimode optical fiber for single particle/cell detection at multiple points on a microfluidic device with applications to particle/cell counting, velocimetry, size discrimination and the analysis of single cell lysate injections. Lab Chip 2016, 17 (1), 145-155.
Kansas State University
College of Arts & Sciences
https://www.k-state.edu/chem/people/grad-faculty/bossmann/MicrofluidicWelcome.html
Please note: This is a commercial profile