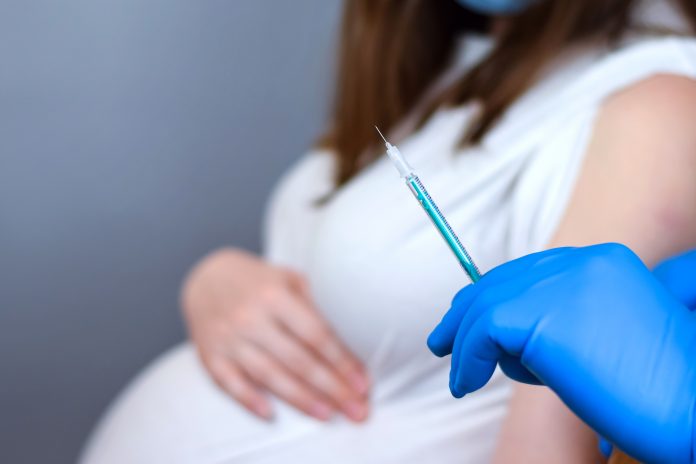
A new clinical trial, funded by the UK government, will investigate the best gap between the first and second COVID-19 vaccine dose for pregnant women
Over 600 pregnant women will participate in a new trial, named Preg-CoV, where they will be given either the Pfizer/BioNTech or the Moderna vaccine to investigate the best dose interval between first and second doses.
Participants will need to be between 18 and 44-years-old, have no health conditions and be between 13 and 34 weeks pregnant on the day of vaccination.
They will receive 2 doses of a COVID-19 vaccine at either the shorter interval of 4 to 6 weeks or the longer interval of 8 to 12 weeks and will be required to attend 9 visits and complete an electronic diary in between on any symptoms.
The scientists will analyse blood samples from the participants and one blood sample from their newborn babies, alongside samples from breastmilk.
‘Safety of the women is the utmost priority’
Health professionals will closely monitor the women throughout their pregnancy and following the birth. They will also be given a 24-hour mobile number so they can contact one of the trial team at any time if they have concerns.
Chief Investigator and Professor of Paediatric Infectious Diseases at St George’s, University of London, Professor Paul Heath said:
“Tens of thousands of pregnant women have now been vaccinated in both the US and the UK with no safety concerns reported, but we still lack robust, prospective clinical trial data on COVID-19 vaccines in pregnant women. This includes the best schedule to use to maximally protect them against COVID-19.
“We are extremely pleased to commence the Preg-CoV trial, which aims to fill these gaps in our knowledge and will ultimately inform policy recommendations on the optimal use of COVID-19 vaccines in pregnancy.”
The Preg-CoV study has been backed by £7.5 million of government funding.