Sean Lawler, Associate Professor at Brown University, explains solving the riddle when it comes to the cytomegalovirus virus and the lethal brain tumour, glioblastoma
Several published studies have proposed a connection between cytomegalovirus (CMV), a widespread virus in human populations and the lethal brain tumour, glioblastoma. Clinical trials targeting CMV in glioblastoma patients have shown promising results, however, the underlying mechanisms have been mysterious and this has become a controversial area. Recent data continue to support the clinical relevance of CMV and suggest that these approaches may become an important aspect of brain cancer therapy in the foreseeable future. This article will summarise the history of the field and the potential impact of anti-viral therapies on glioblastoma patients.
An estimated 15-20% of human cancer is associated directly with viral causation. These virus-driven cancers are specific for tissue type and virus type and can be prevented by prophylactic intervention with anti-viral vaccines or drugs. For example, the application of widespread vaccination against Human Papillomavirus (HPV) will have a major impact on the future incidence of cervical cancer. This is because HPV can drive the transformation of normal ovarian cells to become cancer cells, and the mechanisms involved have been clearly shown. However, the link between viruses and cancer is complex, with multiple mechanisms involved, including the direct transformation of normal cells by virus genes to the promotion of inflammation and genomic instability in virus-infected tissues that ultimately leads to cancer formation and growth. An example of this is the relationship between chronic Hepatitis C virus infection and liver cancer.
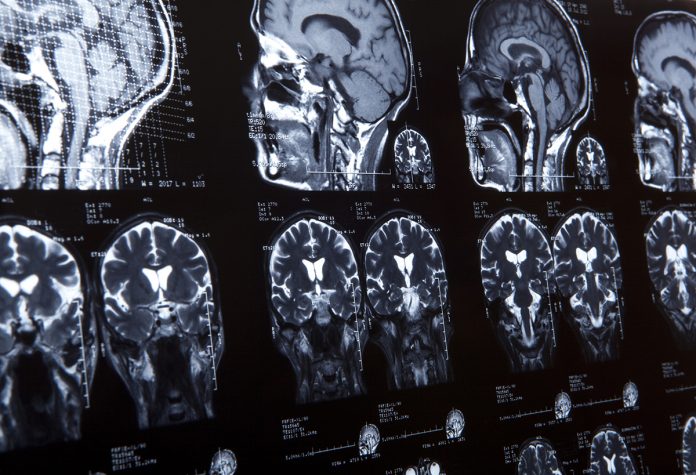
The majority of tumours have no known causal connection with viruses. For example, glioblastoma is a devastating brain tumour with no known cure and cause. Many investigators have attempted to identify viral associations in glioblastoma, but none have been found, with the exception of CMV which has been detected in glioblastoma tumour specimens. CMV is widespread in human populations where it can cause serious problems in the context of organ transplant and is a major public health concern in newborns where infection can affect hearing and neurodevelopmental processes. After initial infection, CMV remains in the host for life in a latent state, where it is essentially dormant, but can re-emerge under certain conditions and also has profound effects on the immune system in ageing hosts.
Potential for association between CMV & glioblastoma
The prevalence of CMV in Western countries is generally about 50%-60%. One of the most interesting and puzzling observations in recent times has been the potential for an association between CMV and glioblastoma. There is no evidence that CMV is causative of glioblastoma, however, drugs and other therapies that target CMV seem to improve outcomes for glioblastoma patients. An initial report in the New England Journal in the 2000s suggested that a patient who was highly responsive to a dendritic cell vaccine was also expressing high levels of CMV in their tumour.
Another series of studies using the anti-viral drug valganciclovir alongside standard of care (surgery, irradiation and chemotherapy) indicate a significant improvement in median survival in glioblastoma patients. A definitive Phase 3 trial is underway at the Karolinska Institute in Stockholm, Sweden. If the results are positive, valganciclovir could likely become a component of the standard of care for glioblastoma. New research is uncovering potential reasons for these observations and revealing subtle but important effects of host-pathogen relationships and cancer growth and therapy.
An association between CMV and glioblastoma was first suggested in 2002 when it was shown that CMV proteins and DNA could be detected in human glioblastoma specimens. This observation spawned a number of studies over the next several years that showed CMV in tumour samples and that various genes expressed by CMV infected cells could promote the growth of tumour cells in the lab. However, other recent studies have failed to confirm the presence of CMV in glioblastoma specimens, and this is an area of debate at present. It may be explained by technical issues to do with sample preparation or sensitivity or because that CMV is not present in tumours at very high levels.
It should also be noted that CMV infection can have profound effects on immune cell behaviour and functionality, particularly during ageing. Because glioblastoma is intimately connected with ageing and the immune system (a substantial portion of the tumour is made up of immune cells), then it is easy to imagine that changes in the behaviour of immune cells due to CMV infection status could be reflected in altered tumour growth patterns.
CMV: An important therapeutic target
Regardless of the debate over the presence of CMV in glioblastoma tumours, clinical evidence in patients supports CMV as an important therapeutic target. In addition to the clinical trials just mentioned, patients with circulating CMV antibodies in their blood (indicative of prior infection) have a small but significant reduction in their overall survival compared with seronegative patients.
Another series of studies have shown that a subset of patients with glioblastoma undergo reactivation of CMV during treatment leading to encephalopathy, which results in rapid decline and reduced survival. This reactivation is likely a result of the therapy that the patients receive, which is a combination of radiation and chemotherapy. Thus, a series of clinical observations as well as the clinical trial data suggest that targeting CMV in glioblastoma could be therapeutically relevant.
To investigate this, my lab developed a model of glioblastoma in which tumour growth is measured in mice infected with CMV. In this model, we found that tumours grow faster when implanted into animals with latent CMV (where the virus is present, but not active) and this can be reversed with an anti-viral drug, supporting clinical trial data. These studies also revealed that CMV is reactivated within the tumour and that its replication is required for the effects on tumour growth. We also observed a pronounced elevation in the number of blood vessels within the tumour which are needed to sustain tumour growth. More detailed analysis revealed heightened inflammation within tumours in CMV mice, with more immune cell infiltrates, and more invasion of the normal brain. These studies in animal models are allowing us to develop a mechanistic understanding of the role of CMV in glioblastoma, providing conceptual support for clinical data, and potentially allowing the development of improved therapies, by developing further anti-viral approaches identifying specific vulnerabilities, which are not seen in the uninfected animals used as standard in the field.
Closing thoughts
Taken together, this combination of lab-based studies, clinical observations and clinical trial data are starting to build a picture where CMV acts, perhaps not as a cancer-causing virus, but as a cancer modulating virus. CMV-infected individuals should be considered as candidates for anti-viral therapy, which based on emerging evidence, will improve patient survival and have an impact on the progression of this catastrophic disease.
References
- Stragliotto G, Pantalone MR, Rahbar A, et al. Valganciclovir as Add-on to Standard Therapy in Glioblastoma Patients. Clin Cancer Res. 2020;26:4031-4039.
- Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347-3350.
- Lawler, S. Cytomegalovirus and glioblastoma; controversies and opportunities. J. Neurooncol. 2015;123:465-471.
- Krenzlin, H, Behera P, Lorenz V, et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Invest. 2019;130:1671-1683.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.