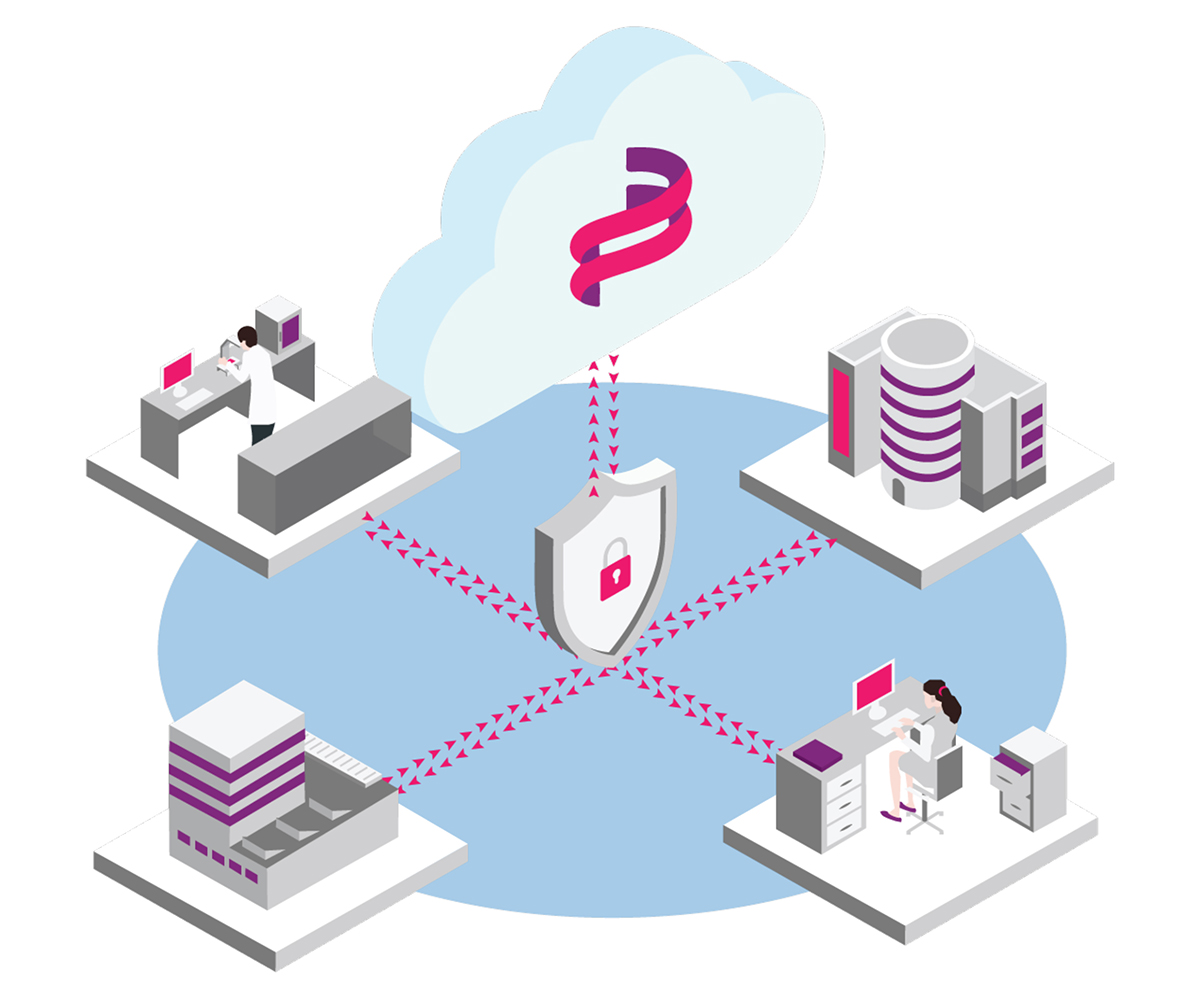
Rapidly scaling health-tech player in the emerging Pathology Computer Aided Diagnostics sector Deciphex uses digital pathology and AI to accelerate pathology diagnosis by improving productivity and patient outcome
We help pathologists focus on the critical disease content, improving clinical turnaround time and patient confidence. Cellular pathology is the diagnosis of disease in tissue using conventional light microscopy, traditionally a practice that has not changed fundamentally in over 150 years. Pathologists are at the heart of the diagnostic process, in particular, cancer diagnostics. Growing pressure on the discipline is driving longer turnaround times for diagnoses and the potential for increased diagnostic error. This ultimately keeps patients waiting for critical news and erodes confidence in the final diagnosis that is provided.
The combination of the increase in cancer incidence, increase in diagnostic complexity, set against declining global pathologist numbers and the increasing demand for specialisation, are pushing the sector towards a crunch point in a ten-year time horizon, unless pathologist productivity can be increased.
Deciphex was founded in 2017 and launched its first product for preclinical pathology in January 2019, Patholytix Preclinical via a collaboration with Janssen and AstraZeneca. Since then, the company has driven considerable traction for its flagship product.
Patholytix Preclinical as a pathology solution
Patholytix Preclinical is a Hybrid-SaaS digital pathology platform designed to facilitate broader utilisation of whole slide imaging and related data in routine non-clinical applications. The platform enables utilisation through design principles closely aligned to the specific needs of the non-clinical pathology market. This includes secure inter-organisational collaboration, end-to-end security, flexibility in-study format & streamlined image viewing, offering equivalent viewing experience on par with traditional microscopy. Patholytix is also the only solution to enable GLP-Peer Review of non-clinical (drug safety) studies.
Patholytix AI
One of the key drivers for the adoption of Digital Pathology is the opportunity to leverage Artificial Intelligence/Machine Learning to drive workflow efficiencies through automated screening of studies to provide decision support to the pathologist. Through a combination of ergonomic user interface & training strategies that require minimal data input, Patholytix AI delivers a simple and efficient workflow that helps reduce the barriers to adopting AI for routine applications.
Deciphex provides a fully cloud-hosted AI service, eliminating the need for any local processing capabilities, as well as a consultancy service via which their in-house experts help you create models tailored to your specific needs and endpoints. These powerful model outputs with integrated AI tools such as Quantitative Pathology, are immediately accessible across your organisation through the Patholytix Study Browser, helping you reach critical decisions more quickly, and with greater confidence.
Global and local challenges in clinical service delivery
Major supply and demand dynamics exist internationally in pathology services. In almost every jurisdiction worldwide the number of required pathologists outweighs the actual number of pathologists and this is steadily increasing, putting significant pressure on pathology services.
In the Clinical space, we are focused on addressing these recruitment pressure points, alleviating wait times, and providing the pre-requisite expertise to ensure the most expeditious and efficacious treatment of patients.
Diagnexia Diagnostics Service: Virtual pathology department
Diagnexia is a novel marketplace that connects hospitals around the world to a global network of renowned subspeciality pathologists using digital pathology, supporting the highest quality of care and underpinning diagnostic turnaround time objectives. Diagnexia opens access to international customers to expertise that they simply would not be able to acquire otherwise.
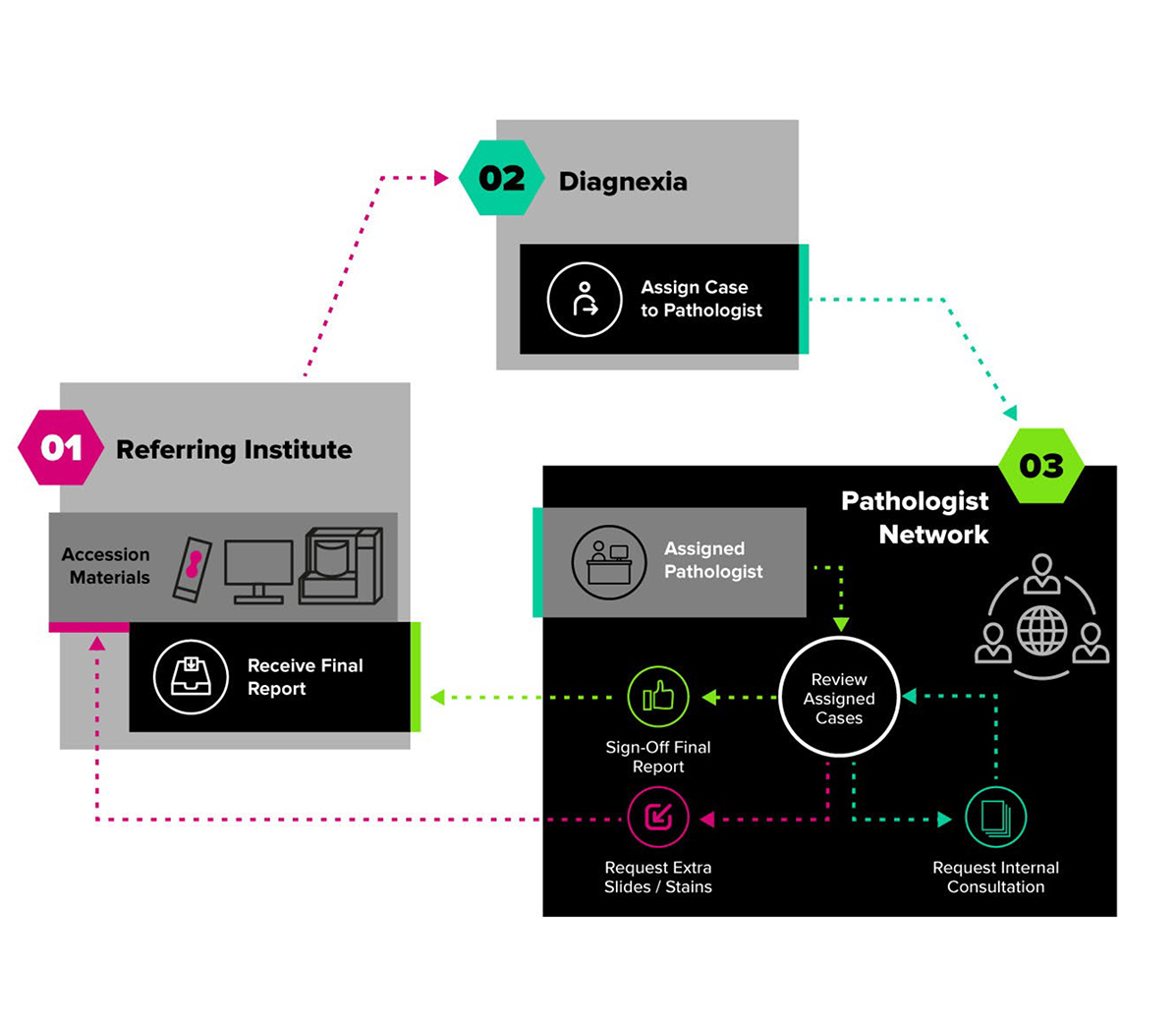
We have developed a state-of-the-art viewing client and pathologist portal using proprietary software that enables an end-to-end seamless, efficient, digital solution incorporating useful AI tools to generate aesthetically clear contemporary pathology reports that are delivered to clients electronically. In other words, we have created a virtual department of pathologists using an industry-leading digital pathology workflow to provide clinical pathology diagnostics.
Services provided
We are growing the pool of diagnostic expertise available to the UK pathology market, through the digital extension of the pathology workforce. Providing a plug and play solution for digital pathology, that opens up access to internationally renowned expertise and enables laboratories to manage their backlog in a secure and efficient manner, allowing for a significant reduction in administration and turnaround time.
Diagnexia pathologists provide three main service offerings to support industry needs.
Primary reporting: Through our accredited laboratory facilities and in markets where regulated digital pathology is allowed, we provide a complete pathology diagnostics service for primary reporting. Primary reporting is provided to enable sustained remote locum services for clients.
Secondary reporting: Diagnexia enables international clients to achieve rapid and reliable secondary consultation on challenging cases. Our expertise in pathology, along with the ability to facilitate up-to-date molecular diagnostics, allows us to deliver a unique solution to facilitate subspeciality consultation.
Quality assurance: Provision of expert unbiased feedback on both diagnostic and technical quality within a facility as part of external facilities quality assurance processes.
Diagnexia pathology team
Diagnexias’ Chief Medical Officer-Prof Runjan Chetty is a highly experienced Anatomical Pathologist with considerable clinical experience, who leads a team of over twenty GMC-registered subspeciality pathologists focusing on the top ten subspecialities.
AI model development and validation for clinical use cases
The key drivers for our clinical AI research activities stem from time-consuming workflow inefficiencies voiced to Deciphex by clinical pathologists. Deciphex has the capability to streamline and automate these processes. Not only can Deciphex automate more tedious tasks, but our technology also has the potential to drive down the cost of clinical slide analysis by making the diagnostic review process more efficient.
Our vision is to integrate artificial intelligence tools to further reduce reporting turnaround times and in turn, result in better patient care. We are using advanced AI tools for feature identification, qualitative and quantitative assessment and prognostic stratification to automate, ameliorate and improve routine clinical diagnostic workflows.
The future
Our overall goal is to streamline and transform digital pathology workflows to make the life of the pathologist easier as well as help pharma accelerate the process of essential drug development and help patients get timely and accurate diagnoses.
Deciphex hopes to become an end-to-end service provider for all digital pathology needs as we integrate more planned features into both our non-clinical solutions and clinical services.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.