Can we prevent the muscle loss associated with some cancers? Dr Vera Mazurak at the University of Alberta is looking into one method of treating myosteatosis
Pathological fat infiltration into muscle is a feature of disease-induced muscle loss that significantly associates with shorter survival in people with cancer. Fat is associated with skeletal muscles in the form of intra-myocellular lipid droplets within the cytoplasm of myocytes, as well as intermuscular adipocytes. These lipid stores are thought to provide fuels for skeletal muscle contraction; however, excess deposition of triglycerides within cells and organs which normally contain only small amounts of fat (such as liver, pancreas, skeletal and cardiac muscle) is defined as steatosis. Myosteatosis (steatosis of the muscle) is a pathological phenomenon reflecting an impairment of synthesis and elimination of triglyceride.
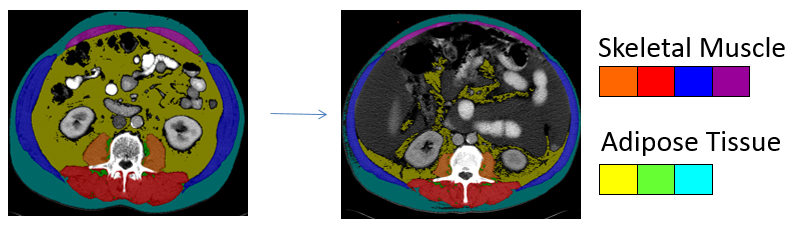
Fig. 1: To quantify different tissues for body composition analysis using computed tomography imaging, a bony landmark is used to consistently measure the same region of the body across patients. The third lumbar vertebrae is an established landmark in body composition analysis that correlates with amount of whole body muscle and fat. Each tissue attenuates radiation in a specific way which is recognised by a software program to enable skeletal muscles and different types of adipose tissues to be identified. Each tissue of interest is then colour coded. When more than one CT image exists in the patient record, tissue changes over the trajectory of the disease can be determined. This image presents two scans taken approximately six months apart at the same region within the same patient. The marked decline in muscle and adipose tissue is evident, concurrent with deposition of adipose tissue into muscle.
Myosteatosis is revealed in vivo by computed tomography (CT) imaging as muscle with low radio-density combined with presence of intermuscular adipose tissue. The evidence for a relationship between low muscle radio-density and shorter survival in people with cancer is building. Loss of skeletal muscle mass appears to generally occur with accumulation of adipose tissue into muscle. We reported that patients undergoing treatment for lung cancer lost muscle mass and concurrently gained intermuscular adipose tissue during treatment for cancer, whereas patients who supplemented their daily intake with fish oil containing eicosapentaenoic acid and docosahexaenoic acid [EPA+DHA (2.2 g/day)] maintained or gained muscle mass and experienced a decline in intermuscular adipose tissue over the same time period. This intervention also resulted in a greater response by the tumour to the drugs being used to treat the cancer. Therefore, there may be multiple benefits of dietary fish oil to the cancer patient undergoing treatment.
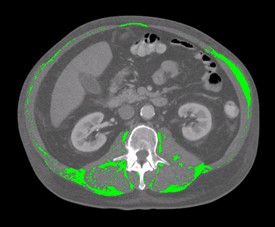
Fig. 2: This slide shows a CT image from a cancer patient who underwent 16 weeks of first line platinum doublet chemotherapy for lung cancer. Only the intramuscular adipose tissue is shown in colour.
Clinical trial expected to verify tantalizing findings
To explore these observations that cancer patients supplementing with EPA+DHA experience an improvement in myosteatosis, we established a preclinical model to enable intervention with EPA+DHA at various time points in the cancer trajectory. We used a rat model bearing the Ward colorectal tumour and treated in a manner that mimics standard clinical care for this disease in humans with respect to the types of drugs used and the toxicities they evoke. Using this model, we have demonstrated that the results align with our human data, suggesting an improvement in muscle condition concurrent with a better response by the tumour to the anti-cancer drugs.
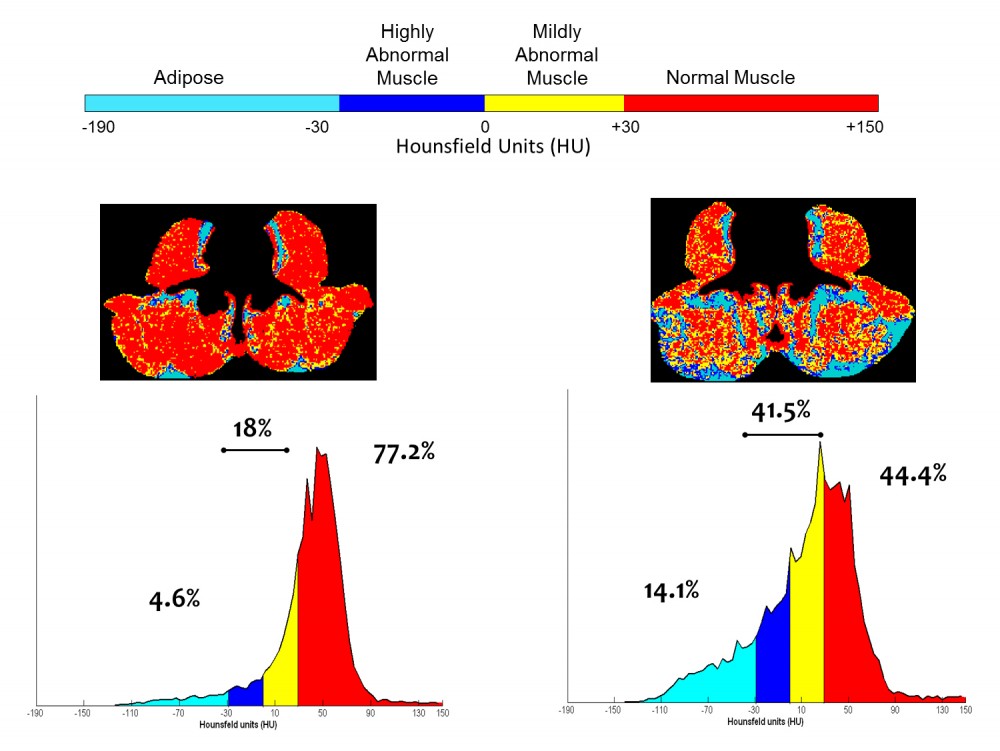
Fig. 3: An illustration of annotated CT images, and accompanying histograms of radiation attenuation showing the percentages of total tissue cross-sectional area within the ranges of adipose tissue in paraspinal/psoas muscles is useful to understand the problem of myosteatosis. This illustration shows the percentages of total tissue cross-sectional area within the typical attenuation ranges determined for the respective tissues for 2 subjects. Subject 1 is a 63 year old male with muscle characteristics at the median values for male cancer patients with a diagnosis of solid tumour at our centre. For Subject 2 there is extensive macroscopic adipose tissue and less than half of the cross sectional area of his muscles falls within the normal attenuation range. Overall, Patient I has a greater proportion of fat and low attenuation muscle, than muscle with normal characteristics. Patient II is remarkable in several respects, including extensive visible fatty infiltration and extremely high proportion of total muscle area falling within a range of attenuation values generally recognized to be abnormally low (adapted from Aubrey et al 2014).
Using this as the rationale for the next step in this line of questioning, we have planned a clinical trial upon which to text the biological efficacy of fish oil to reverse cancer-associated myosteatosis in a cancer population known to exhibit myosteatosis, verified by in vivo imaging of muscle features by CT scan. At the time of diagnosis and treatment planning, patients will be randomised and consented to consume EPA+DHA (2.2 g per day) until day of surgery (at least a four week period) or receive standard of care (no intervention). Muscle from the subjects will be collected at the time of surgery and prepared for analysis.
Analysis of the muscle tissue will enable determination of differences in triglyceride-fatty acid content (a hallmark of myosteatosis). We expect that this research will verify the tantalising evidence we have in hand that suggests an improvement in pathological features of myosteatosis by dietary EPA and DHA. If so demonstrated, this work will provide critical translational knowledge required to effectively plan treatment interventions that have significant potential to impact the lives of people diagnosed with cancer, a major cause of death globally.
Dr Vera Mazurak
University of Alberta
Please note: this is a commercial profile