Friedemann Freund, Professor at the SETI Institute/NASA Ames Research Center provides a fascinating look at an aspect of earth science that concerns the search for the origin of life itself
I, Friedemann Freund, started out studying mineralogy and crystallography, had my first academic position as Assistant Professor in Chemistry at the University of Göttingen in Germany, moved on to a professorship in the Geosciences at the University of Cologne, also in Germany, and came in the mid-1980s to the NASA Ames Research Center in California, U.S., and joined the Physics Department at San Jose State University as an Adjunct Professor.
My interests have always gravitated around defects and impurities in crystals, in particular, those that arise from the interactions of minerals with the common gas-fluid components water, carbon monoxide and dioxide, nitrogen, and sulphur. I discovered that the low-z elements H, C and N (which happen to also be the biogenic elements par excellence, on which life is built) can undergo a redox conversion that leads to an unexpected and new form of organic chemistry. At the same time, oxygen becomes oxidized – a process that introduces a good deal of semiconductor physics and opens a window of opportunity to study earthquakes from a perspective that had never before been explored.
No quest for knowledge is more profound than the search for the origin of life
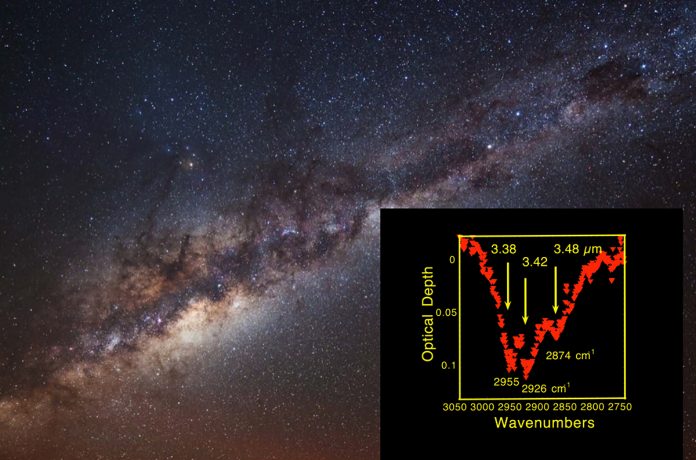
On a clear moonless night, we may look up to the sky and see countless stars. We know that some of the speckles of light are far-away galaxies with their own countless stars. We see the Milky Way – a faintly glowing river of light marking the galactic plane, partly obscured by dark bands. These dark bands are dust clouds spreading through the interstellar space, composed of quadrillions of tiny mineral grains. These mineral grains are chockful of organics. Using a star embedded in a dust cloud as a lamp reveals the presence of aliphatic hydrocarbons, delicate organics that should not be able to survive in the harsh radiation environment of space, surely not for hundreds of millions of years. Yet, these organics are there, undeniably so, as Figure 1 shows – delicate but seemingly indestructible.
…and here on Earth we have life. When Earth formed some 4.5 billion years ago, it was a lifeless orb of rocks and water, shrouded in a thick atmosphere. How could life have ever arisen here? How did it happen?
Over the past hundred years some of the world’s best minds in chemistry, biochemistry, physical chemistry, chemical physics, and astrobiology have pored over this seemingly intractable question. Their goal? To find out how those complex multifunctional organic macromolecules might have formed that were needed for even the simplest life to start.
Tens of thousands of studies have been conducted, focusing on chemical reactions in the gas phase, in the liquid phase, at gas/liquid and gas/solid interfaces, even within the layers of soft clay minerals. None of them produced the insight needed to understand how atoms of carbon, hydrogen, oxygen, nitrogen and sulphur could have combined on the barren early Earth to form the CHONS macromolecules necessary for life to start. The brutal truth is slowly sinking in that understanding the origin of life may still be quite a way off.
Something fundamentally new was needed, something different. This is where my work comes in.
At the beginning, I did not set out to study the origin of life. Far from this esoteric aspiration, I just wanted to find out how the common magmatic gases deep in the Earth – water, carbon dioxide, nitrogen and sulphur compounds – interact with the minerals that crystallize deep down.
I was in for a big surprise. To explain it, I have to become a bit technical.
When I started this work, it was well known and accepted in the science community that minerals, which gobble up magmatic gases such as water and carbon dioxide during crystallization but have no good place to put them in their solid matrix, rip these molecules apart and incorporate them as hydroxyl and carboxy anion impurities. No one suspected, however, that this was not the whole story. My work led me to recognise that, at one point upon cooling, the protons in those hydroxyls and the carbon in the carboxy anions would steal electrons from their oxygens. As a result, the protons turn into hydrogen and carbon atoms turn into chemically reduced carbon – “organic” carbon – just like that, by a purely physical process.
The follow-on steps were even more baffling: the oxygens that had given away an electron changed their bonding character. They become highly deformable, allowing the chemically reduced carbon atoms to diffuse with relative ease through dense mineral structures, even when classical diffusion theory said that they should stay put. Indeed, I found the carbon atoms to be unexpectedly mobile, able to segregate to places, where they can ease the local stresses which they produce. Other carbon atoms do the same. Thus, carbon atoms come in close contact with each other and start tying carbon-carbon bonds.
Hydrogen molecules join in, tying carbon-hydrogen bonds. As a result, organic protomolecules form in the most unlikely place in the world: inside the seemingly forbidding densest mineral structures.


from crushed olivine single crystal grown deep in the Earth

still make up most of the sand
Organic synthesis in the solid state
As so often in science, when something is discovered which seems to be fundamentally new, a torrent of disapproval arises among specialists, many of whom said that this can’t be true and trying to disprove the findings. This happened to me with such vehemence that – for some time – I retreated from this field altogether to let the controversy die down.
But, of course, I could not let go. Often my thoughts returned to this nagging idea, whether this organic synthesis in the solid state may help us find an answer to humanity’s most profound question: Where are we coming from and are we alone?
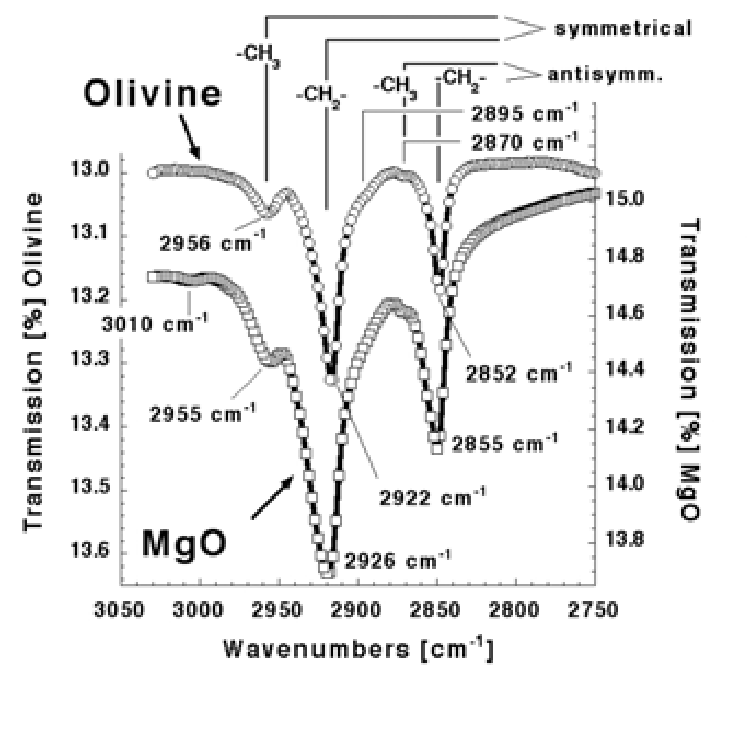
The tiny dust grains in the interstellar dust clouds consist mostly of olivine, a mineral that we also know on Earth. The dust grains are chockful of organics. If we take a gem-quality olivine crystal from Earth and shine an infrared beam through it, we see essentially the same organic signature as Figure 2 documents. This olivine crystal has grown in a searingly hot magma deep below. Yet, in its matrix, there are delicate organics. When we go into the laboratory and grow magnesium oxide crystals from their more than 2600°C hot melt, we see the same spectroscopic signature.
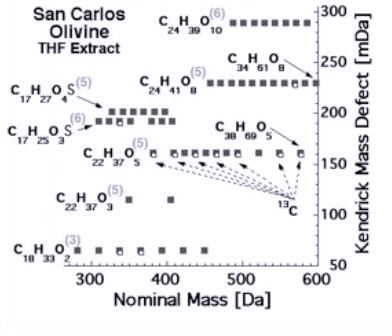
When we take such crystals and crush them to expose those internal organics, we can extract whole families of CHONS with molecular weights up to 600 atomic masses as reported in Figure 3a. In addition, these CHONS are multifunctional macromolecules – just as needed.
When the Earth was young, olivine was abundantly available at the surface, exposed to rain and all other forms of erosion. On many beaches, the sand consisted of olivine as in this rare green beach in Hawaii depicted in Figure 3b. As the olivine crystals weathered away, they must have released their load of multifunctional macromolecular CHONS into the surface waters. Charles Darwin suggested in 1871 that life might have started in some “warm little ponds”.
Yes, Darwin was probably right, but if my work on organic synthesis in the solid state is on target, the organics in “warm little ponds” that crossed the barrier from lifeless to life were not delivered by meteorite impacts from outer space nor generated by electric discharges in Earth’s atmosphere. They were most likely released by gentle weathering from the matrix of minerals such as olivine that had gobbled up water, carbon dioxide, nitrogen and sulphur at high temperatures deep in the rock column and created – upon cooling – precursors of amazing, probably life-giving CHONS.
*Please note: this is a commercial profile