Friedemann Freund, Professor at SETI Institute/NASA Ames Research Center, explains a physics-related question within the field of earth sciences that concerns the unsettled peroxy story
It’s time to address some of the more physics-related questions. The reason why peroxy can be introduced into rocks deep in the Earth’s crust is easy to understand: It’s hot down there. The minerals that crystallise or recrystallise under these conditions invariably gobble up small amounts of H2O in the form of hydroxyls such as, for instance, O3Si-OH. Many hydroxyls form pairs and those pairs can react in two ways: either dehydrate, splitting off H2O or – more riveting – rearrange in such a way that the hydroxyl protons “steal” an electron from their respective oxygens. The two protons turn into an H2 molecule, while the two oxygens change their valence from 2- to 1- and form a peroxy bond. It’s called a “redox conversion”. 125
Why are peroxy bonds important?
There are huge numbers of peroxy in the rocks that form the Earth’s crust. Every pound is estimated to contain about 1021. Yet, because peroxy bonds are inconspicuous, their presence has gone unnoticed for a very long time. Only when something “nasty” is done to the rocks will their peroxy manifest themselves in detectable ways. For instance, mechanical stress will shift mineral grains relative to each. Peroxy bonds that straddle grain boundaries can then break, activating electrons and holes – the two types of electronic charge carriers of semiconductor fame. What is different in the case of rocks is that the electrons get trapped in the broken peroxy bonds, while the holes are free to roam around – even beyond the boundaries of the rocks that are being stressed. 127
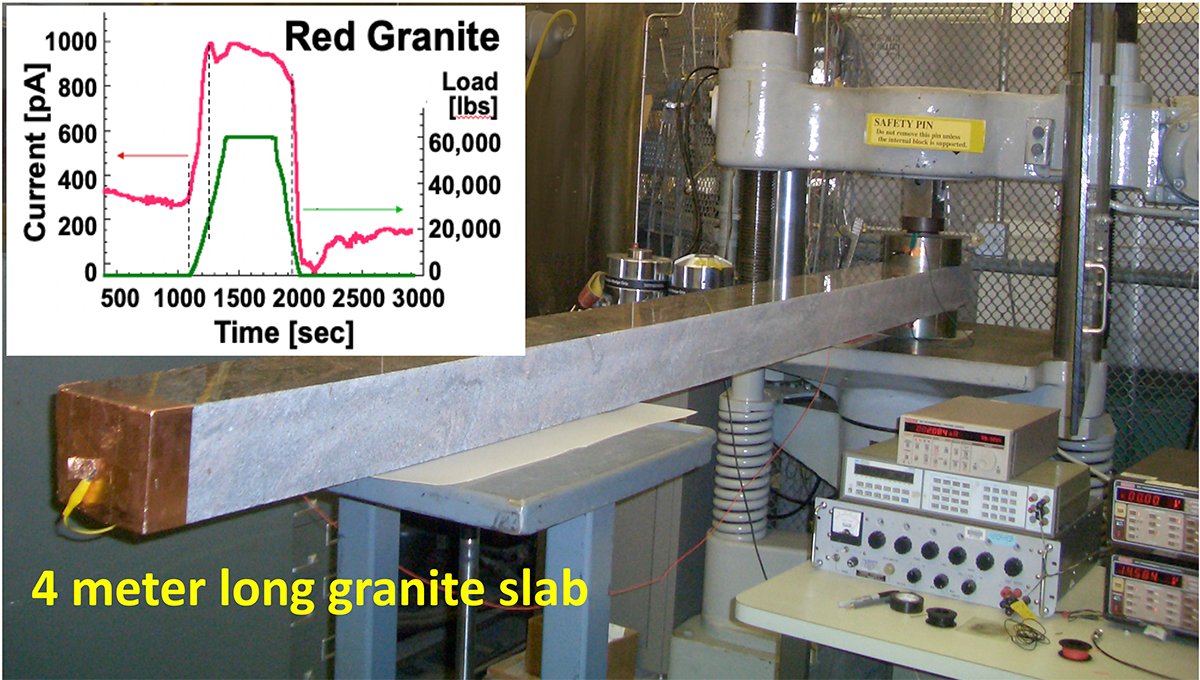
This whole process is surprisingly easy to demonstrate in the laboratory as Figure 1 illustrates. Just take a rectangular chunk of rock, any size, slab on copper electrodes at the far end, where the rock will be stressed and at the unstressed near end. Run a wire between the two copper contacts, insert an ammeter and start to stress the far end. As soon as a load is applied (green curve in the insert), an electric current begins to flow (red curve). The current rises sharply with the load, passes through some wiggles as the load still increases, saturates and slowly decreases with time, as the load is held constant.
By changing the stress rate from fast to slow, covering more than five orders of magnitude, we can obtain information about the lifetimes of the stress-activated electrons and holes. By keeping the load constant, we learn how long the holes hang around before returning to the peroxy state. The lifetimes thus obtained range from milliseconds to several months. 168
What did the discovery of peroxy tell us?
The discovery of peroxy in the rocks that form the Earth’s crust has led to many new insights. One obvious connection is to earthquake science. Hole-type charge carriers activated prior to major earthquakes stream out of the stressed rock volumes, travelling fast and far. Along the way, they generate electromagnetic waves. They accumulate at the Earth surface, setting up microscopic but steep electric fields, high enough to field-ionize air molecules, in particular, O2, to O2+ as illustrated on the right side of Figure 2. The positive air ions rise through the troposphere into the stratosphere, perturbing the ionospheric plasma by pulling down its electrons and causing anomalies in the so-called “Total Electron Content”, TEC. 114
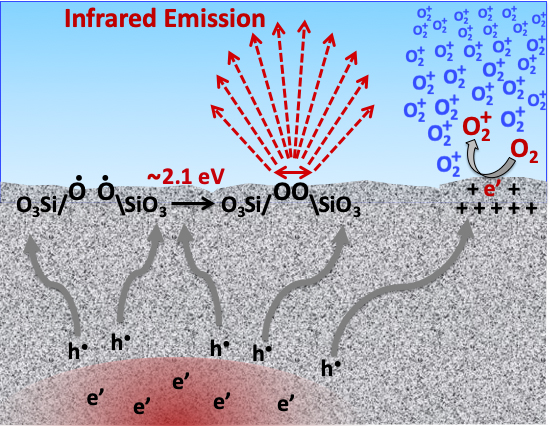
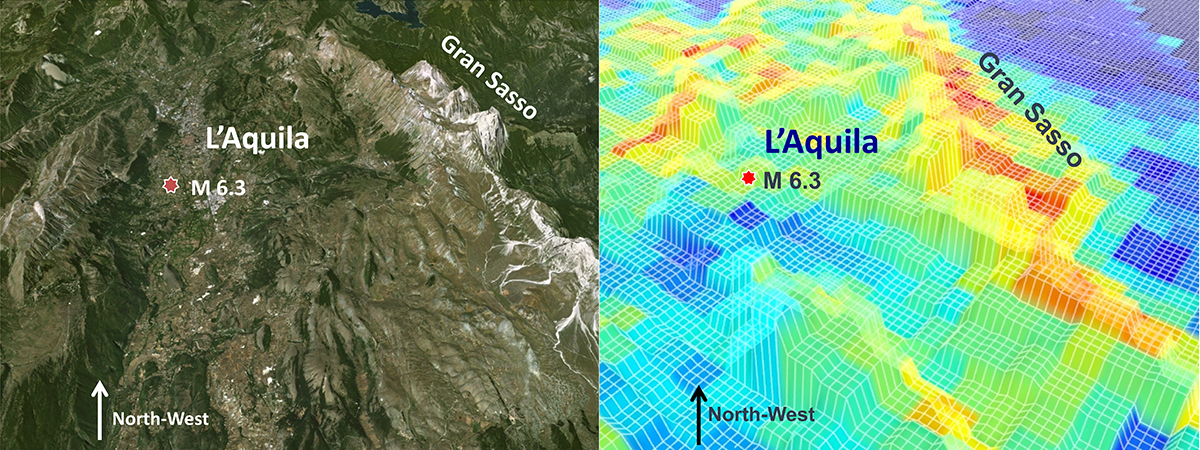
The hole charge carriers also tend to accumulate at topographic highs, such as mountain tops. Returning to the peroxy state as illustrated on the left side of Figure 2, the newly forming peroxy bonds are vibrationally very “hot”, roughly 20,000 degrees Kelvin. They emit extra infrared photons in the so-called “thermal” region. Satellites then record “thermal infrared” (TIR) anomalies as documented in Figure 3, which depicts (left) the valley of L’Aquila in central Italy, flanked by mountains including the Gran Sasso d‘Italia, the highest peak in Italy south of the Alps. The TIR image (right) was obtained three nights before the deadly 2009 magnitude 6.3 earthquake that destroyed large parts of the medieval town of L’Aquila. During that night, the mountain tops on either side of the valley were glowing in the infrared as if they were 15°-20° degrees Celsius hotter than normal, but it wasn’t “heat”. 146
The discovery of peroxy in the Earth’s crust may be opening a new era in the Earth sciences that will help decipher many processes in our natural environment that are still unsettled. 33
*Please note: This is a commercial profile