Dr Carlos Ziebert’s team, of the Karlsruhe Institute of Technology (KIT), explores the safety of batteries across calorimetric studies
Work
The group Batteries – Calorimetry and Safety focuses on calorimetric studies and safety tests on lithiumion cells and postlithium cells. For this purpose, depending on the cell size and application, different types of calorimeters are used in Europe’s largest Battery Calorimeter Laboratory, which was established in 2011. As shown in Figure 1 It provides seven Accelerating Rate Calorimeters (ARCs) from Thermal Hazard Technology, allowing the evaluation of thermodynamic, thermal and safety data for Lithiumion and postLi cells on material, cell and pack level for both normal and abuse conditions (thermal, electrical, mechanical). The lab also includes glove boxes for the cell assembly and disassembly, many temperature chambers, a thermal camera and cyclers with several hundred channels. In addition, it contains extremely sensitive 3D Calvet calorimeters, which provide thermodynamic parameters and gas chromatographymass spectrometry systems from PerkinElmer for venting gas analysis.
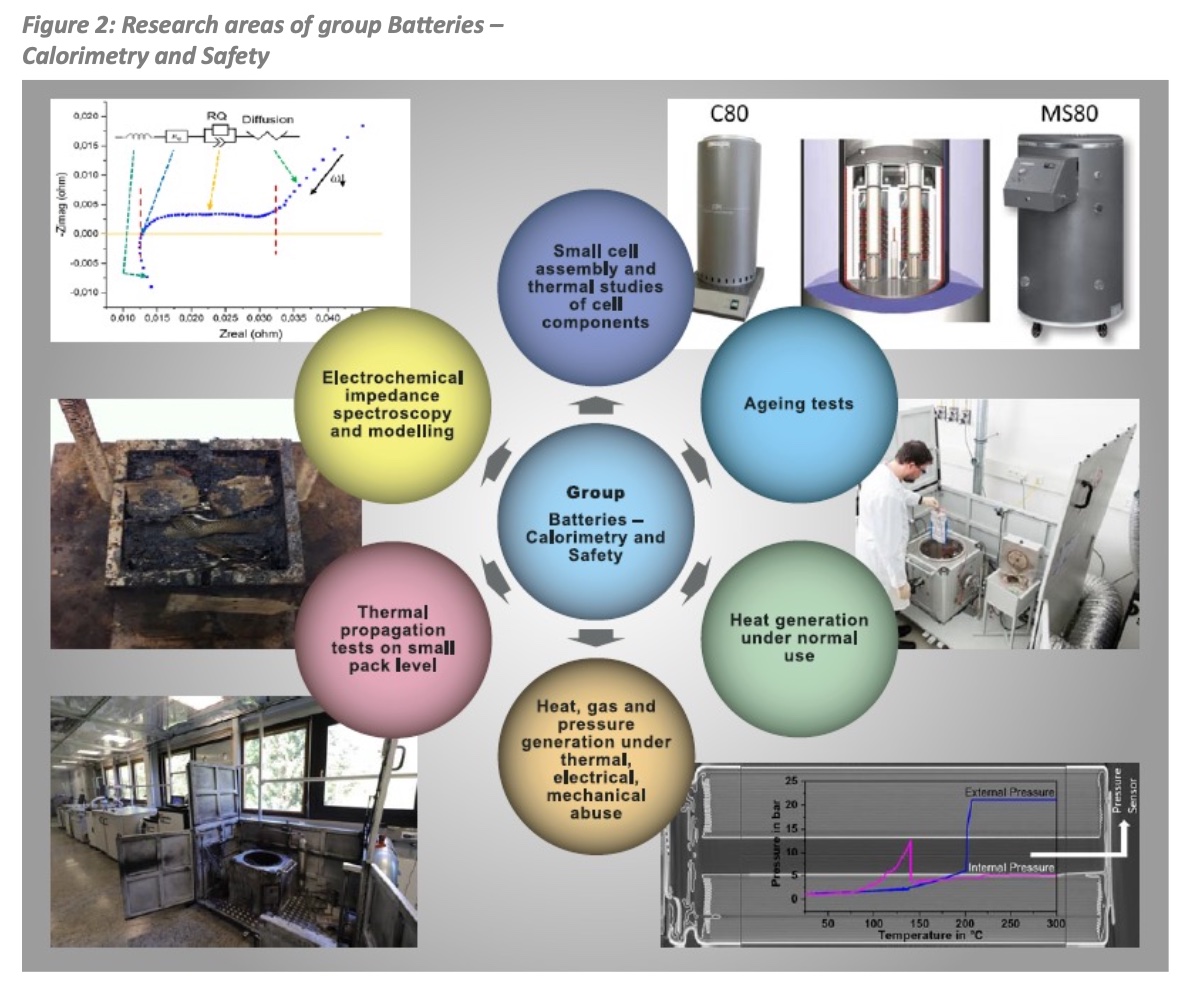
The group’s work can be categorized into the following six areas as illustrated in Figure 2.
- Small cell assembly and thermal studies of cell components
- Ageing tests
- Heat generation under normal use
- Heat, gas and pressure generation under thermal, electrical, mechanical abuse
- Thermal propagation tests on small pack level
- Electrochemical impedance spectroscopy and modelling.
Projects
The group is interdisciplinary and wellconnected across both Germany and Europe and pursues a comprehensive approach that ranges from basic studies to industrial applications. Starting with four members in February 2020, the group has been growing up to fifteen members due to participation in three national and European projects in Horizon 2020.
AnaLiBa
The first national project, which has started on the 01.01.2021 is called AnaLiBa (Analytics of LithiumIonBatteries) [1] in the competence cluster Analytics/quality assurance (AQua). It is funded with about 1.3 million Euro by the German Federal Ministry of Education and Research (BMBF) in the framework of the competence cluster Analytics/Quality assurance (AQua). The project is coordinated by the Fraunhofer Institute for Chemical Technology (ICT) in Pfinztal, and the second partner is the Centre for Solar Energy and Hydrogen Research BadenWürttemberg (ZSW) in Ulm. In the AnaLiBa project, the main aim is the joint method development of a standardised sample handling process for the gas analysis of large cells. This encompasses at first a method in which a pressuretight canister is connected via a small capillary to a gas mouse, at second the direct insertion of the capillary into the cell and at third a direct coupling of the ARC and an online mass spectrometer. Knowledge of the gas composition under the various cell abuse scenarios reveals the relevant decomposition paths and gives an idea, which protective measures are necessary, for example, to delay or prevent the cell fire caused by the ignition of vent gases or thermal runaway.
In the project both different cell formats, different cell capacities and different areas of application (highenergy/highperformance cells) will be compared.
BatgasMod
The second national project is called BatgasMod (Modelling of Battery Gasing) [2] and has started at the 01.10.2021. It belongs to the new battery competence cluster Battery usage concepts (BattUse). This competence cluster BattUse aims to foster our understanding of battery behavior to determine when the second use of battery storage is preferable and is funded by the German Federal Ministry of Education and Research – BMBF by 20 million Euro. The reactions of the liquid electrolyte and the associated gas formation play a decisive role in cell ageing and cell safety. These are, therefore the focus of the BatgasMod project funded by the BMBF with around 1.3 million euros. It is led by the IAMAWP at KIT, other partners are the Institute for Power Electronics and Electrical Drives (ISEA) at RWTH Aachen University and the Münster Electrochemical Energy Technology (MEET) at the University of Münster. The aim of the project is to develop electrolyte aging models in combination with battery models for the early prediction of cell behavior in the usage phase.
POLIS
As part of the Excellence Initiative, the KIT, the University of Ulm, the Center for Solar Energy and Hydrogen Research BadenWürttemberg, and the University of Giessen jointly launched in 2019 the POLiS – Cluster of Excellence for Battery Research Post Lithium Storage, which is funded with 47 million euros over seven years. Post lithium batteries use more abundant and environmentally friendly materials instead of Lithium, Nickel and Cobalt. These can be Sodium, Magnesium or Calcium. In the first threeanda halfyear funding period, the focus was laid on the development of sodiumion batteries (SIB), which are based on the same working principle as that of a Lithiumion battery. Instead of lithiumions, sodiumions are transferred via an organic electrolyte through a separator between the two electrodes, in which they are intercalated and deintercalated, respectively. In POLiS, the group is responsible for the area of thermal characterization and safety of Sodium and Magnesium ioncells, which are expected to provide higher sustainability, safety and storage capacity combined with lower costs.
HELIOS
In the European project HELIOS (High pErformance moduLar battery packs for sustaInable urban electrOmobility Services), which is funded by around 10 million Euro and has started at the 01.01.2021, improved modular battery packs are developed together with the following 18 partners from Europe and Turkey under the lead of Aarhus university:
- Aarhus University (Denmark)
- Karlsruhe Institute of Technology (Germany)
- Izmir Institute of Technology (Turkey)
- Aalto University (Finland)
- IREC (Spain)
- RDIUP (France)
- NVISION (Spain)
- VESTEL EV Charging Solutions (Turkey)
- VITESCO (Germany)
- IDNEO (Spain)
- BOZANKAYA (Turkey)
- Universitat Politécnica de Catalunya (Spain)
- Center for Solar Energy and Hydrogen Research BadenWürttemberg (Germany)
- Danish Technological Institute (Denmark)
- TU Sofia (Bulgaria)
- TUBITAK MAM (Turkey)
- European Copper Institute (UK)
- KNEIA (Spain).
Applying a holistic circular approach, the HELIOS project investigates optimal ecodesigns and advanced processes to both enhance and demonstrate innovative, lighter and ecofriendly hybrid LiIonbased battery packs for midsize vehicles and city buses.

Battery venting gas analysis using battery calorimeters & gas chromatography in AnaLiBa
Typically cylindrical cells and prismatic hard case cells provide socalled safety vents, which are predetermined breaking points that allow releasing the overpressure that is induced by the gas formed due to an abuse of the cell, such as overheating or overcharging. Pouch cells, in contrast, tend to open at any weakest point of their welding seeds. This can be close to the current collectors, but this point is not as well defined as for the two other cell types. To collect the gases during the venting of the cell, an abuse test is performed in the ARC inside a pressure tight cylinder (see Figure 3). Two approaches for gas collection have been elaborated in our lab:
i) by a syringe and ii) by a gas mouse. The canister’s lid is equipped with a thin burst disc made from brass. After the abuse experiment, this burst disk can be pierced by the syringe’s needle, and gas can be drawn into the syringe. For the second approach, the cylinder is connected via a small capillary to a gas mouse. When the cells vent, the gases are collected in the gas mouse. Directly after the test, the gas mouse is brought to the gas chromatography mass spectrometry (GCMS) system. Then the gas is released via a gas feeding system into the inlet of the GC and is analysed both qualitatively and quantitatively using the three thermal conductivity detectors (TCD). If unknown components are found, they can be further inspected using a mass spectrometer.
Correlation of electrolyte properties, gas formation and heat generation by electrochemical-calorimetric methods in BatgasMod
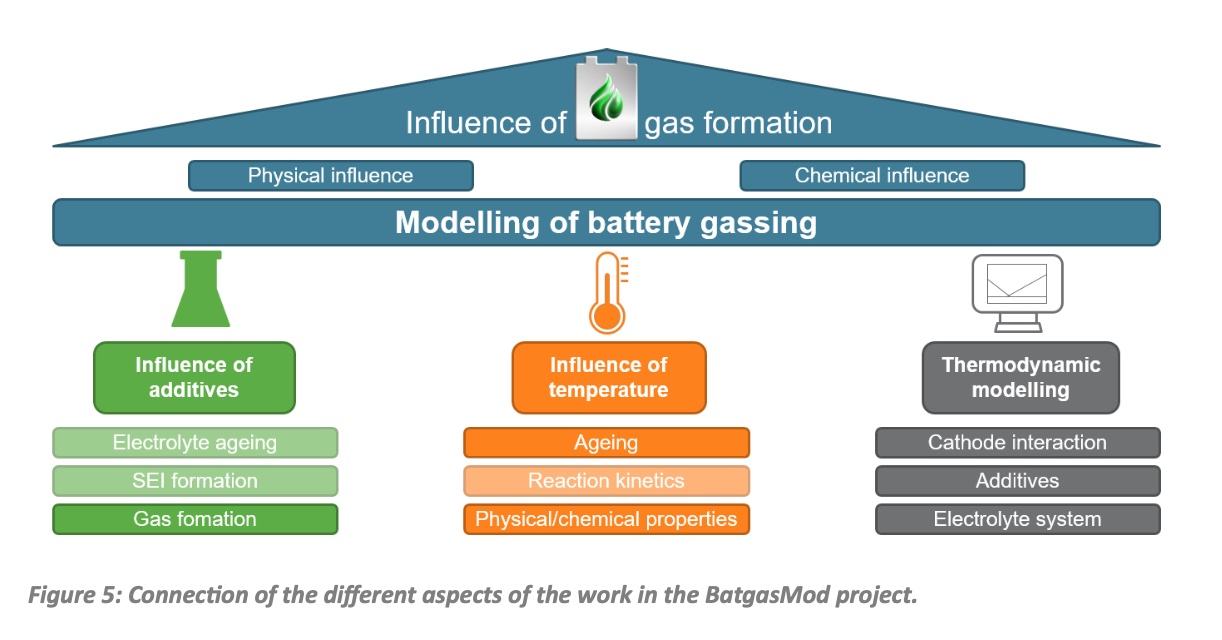
To guarantee a permanently high performance of lithiumion batteries (LIB) in longterm operation requires the perfect interaction of the constituent components, such as electrodes, electrolyte, separator and current collectors. However, extensive material reactions take place during permanent charging and discharging, but also during the initial battery formation, which can adversely affect the behavior of the LIB. It is of cause not possible to completely avoid this degradation of the LIB, but an improved understanding of the different ageing processes helps to reduce the degradation. The main aim of the BatgasMod project is to develop electrolyte aging models in combination with battery models for the early prediction of cell behaviour in the usage phase. Figure 5 illustrates the different aspects of the project. The work at the IAMAWP is particularly highlighted here. It focuses on two areas. On the one side, electrochemical calorimetric measurements are carried out on the active materials and the electrolytes as well as on the cells. This will ensure a consistent modelling in order to reveal the decomposition mechanisms of the electrolyte and the simultaneous buildup of gas pressure during cell use. On the other side, the results of the calorimetric measurements are fed into a thermodynamic modelling of the electrolyte components, the additives and the electrode materials using CALPHAD (CALculation of PHAse Diagrams) methods provided by the ThermoCalc software package. These methods are used to calculate phase diagrams and thermodynamic functions that will be finally implemented in the overall model provided by ISEA. Using this approach safety can be increased and the LIB can be operated much longer, thus enhancing their sustainability.
At first calorimetric investigations of the cell components were performed using highly sensitive TianCalvet calorimeters and differential scanning calorimeters. From these measurements, thermodynamic data of the electrolyte components ethylene carbonate (EC) and ethyl methyl carbonate (EMC) were modelled using the CALPHAD method.
At second pouch cells with a nominal capacity of 5 Ah were manufactured by the MEET battery production line and made available to KIT without a formation procedure. Three cell variants with different electrolyte compositions were produced, namely reference cells with a mixture of the organic solvents EC and EMC in a ratio of 3:7 (LP57) and LiPF6 as conductive salt and cells with 5 wt.% vinylene carbonate (VC) or fluoroethylene carbonate (FEC) as an additive. Li(Ni0.6Mn0.2Co0.2)O2 (NMC 622) is used as cathode and graphite as anode. At third, the pouch cells underwent a formation procedure at the IAMAWP. It was found that the discharge capacity in the first cycle of the cells with additives was larger than that of the reference cells. This can be explained by the intended decomposition of the additives instead of the electrolyte. After formation, the cells were degassed and resealed to remove the gases that formed during formation from the pouch bag.
The gases were analysed qualitatively in a gas chromatographymass spectrometer and typical decomposition products of the electrolyte or the additives such as CO, CO2, methane, ethane and ethene could be found. After resealing and performing additional ten charge and discharge cycles, the heat capacity of selected cells was determined using highly sensitive heat flow sensors (HFX) that were attached to the centre of the pouch cell surface. To evaluate these measurements, an evaluation routine was developed using MATLAB software. Furthermore, isothermal tests were carried out. The cells were cycled in a climate chamber at 25°C with different Crates (0.2C, 0.5C and 1C). During cycling the change in the surface temperature was measured by thermocouples. In addition, a heat flux sensor was attached to the cells to determine the generated heat. As an example, Fig. 2 shows the curves for an Isothermal test on a cell with 5 % VC at 25°C and a 1C charge/discharge rate. First findings indicate that cells with VC additive produce more heat than cells with FEC. Now the different cells will undergo calendaric and cyclic ageing in order to study the influence of the ageing conditions on the gas formation and heat generation.
The overall model finally will make it possible to understand in detail the decomposition of the battery electrolyte in the course of battery use and the effects on aging. In this way, safety can be increased and the LIB can be operated much longer without disruption, which increases their sustainability. It is intended that both novel test methods that are developed in the course of the project and the complete model that includes electrolyte ageing and the related gas formation can be transferred to industrial partners afterwards. By transferring the model to newly developed materials and cells, their timeto market is shortened, which allows the model to check performance and service life with regard to electrolyte aging at an early stage. This serves both the mission of the BattUse cluster and the innovation pipeline of the BMBF umbrella concept for the German battery research factory.
How calorimetry can pave the way for mature and safe sodium-ion batteries in POLiS
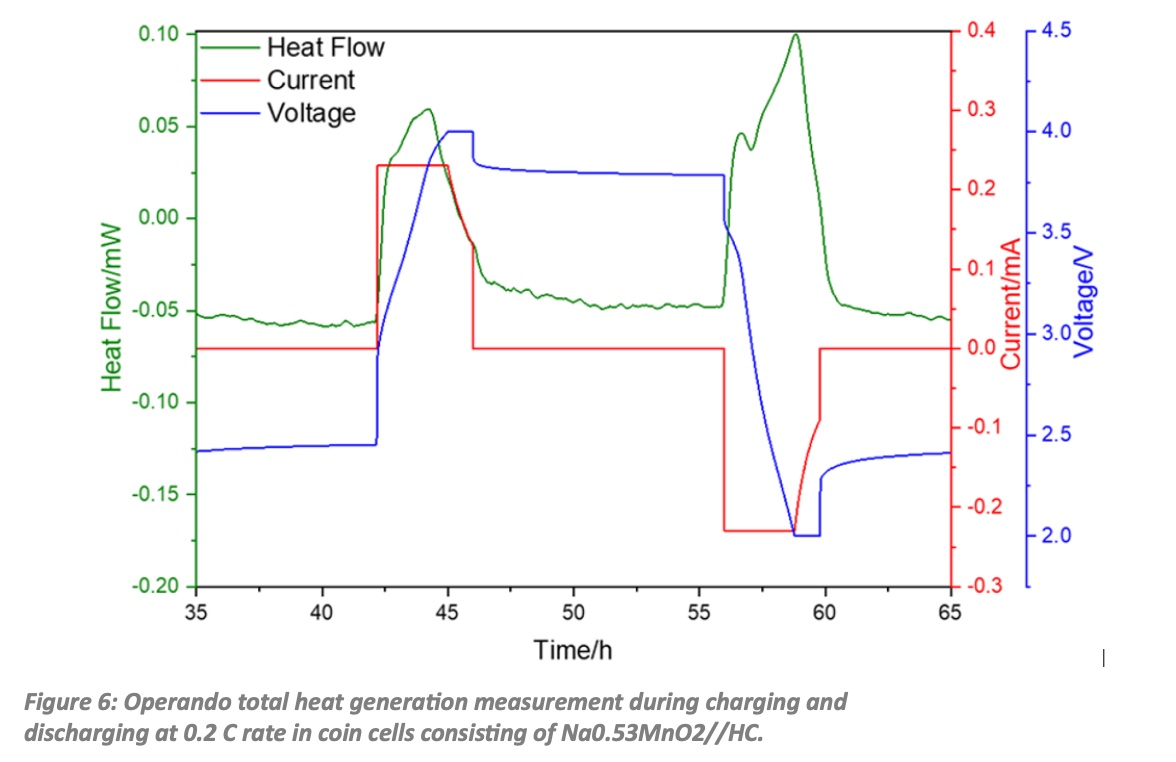
In POLiS the work in the group Batteries – Calorimetry and Safety at the KIT, IAMAWP started with coin cells made of the commercial cathode material Na0.53MnO2 (NMO) and commercial coconutshell derived hard carbon (HC) as anode material. Besides the electrochemical, the thermal characterization is needed to achieve indepth understanding of the underlying reaction mechanisms and heat conduction processes. The MS80 TianCalvet calorimeter allows to determine the total heat generation operando (while operating) during cycling with great accuracy by direct heat flow measurement. The heat flow is determined by the 3D TianCalvet Sensor arrangement, where both the sample and the reference vessel are surrounded by rings with hundreds of thermocouples. As shown in Fig. 6, even if such cells have only a capacity of about 1 mAh the heat flow (green curve) can be clearly measured for the charge and discharge process as indicated by the applied current (red curve) and the resulting voltage (blue curve). For a charge/discharge rate of 0.2 C integration of the heat flow over time gives 1.3 J of generated heat during charging and 1.5 J during discharging.
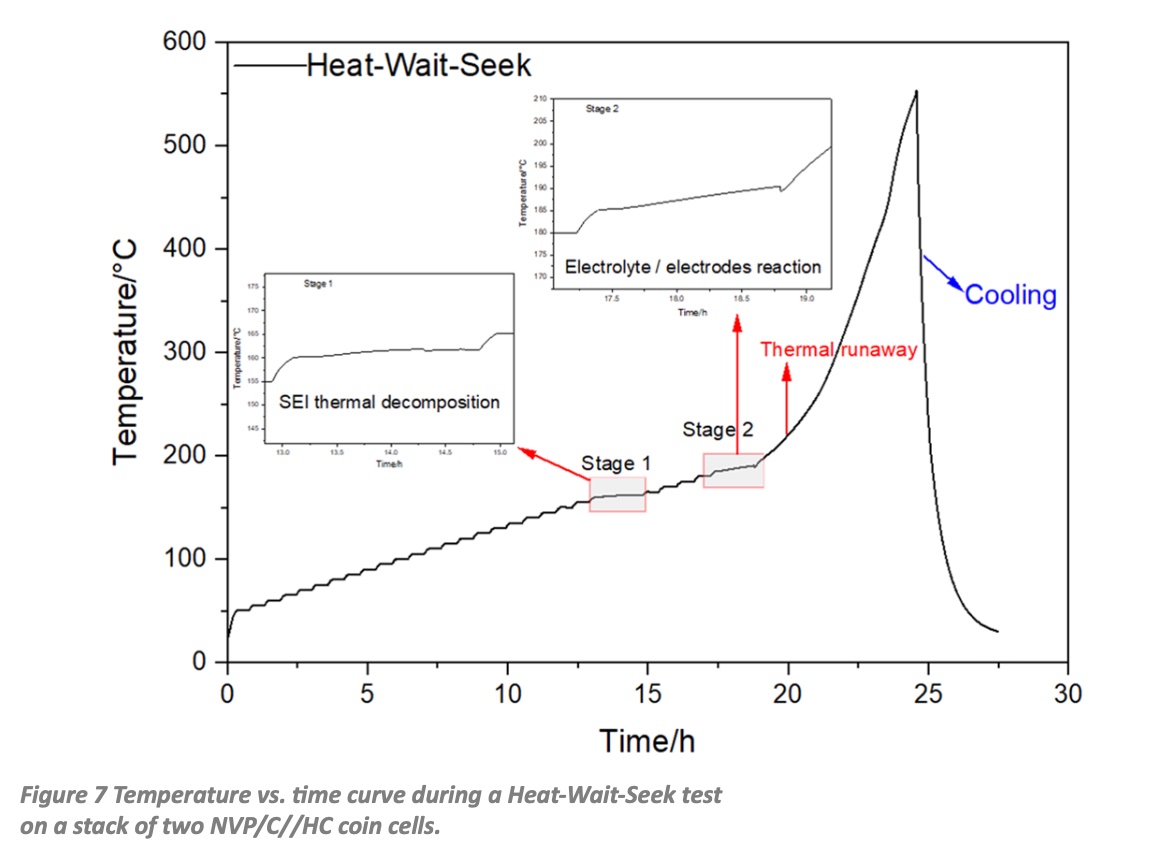
The next step was to replace the NMO by porous Na3V2(PO4)3 (NVP) composites with carbon coating that have been developed at the Institute for Applied Materials – Energy Storage Systems (IAMESS) to enhance the low intrinsic conductivity of NVP by creating an electronic conductive network. Using this improved material the heat generation of a coin cell was largely reduced by a factor of three. For a charge/discharge rate of 0.2 C the generated heat during charging was 0.3 J and during discharging it was 0.5 J. The further scaleup process to pouch cell level needs to be accompanied by safety tests (thermal abuse, overcharging, internal/external short circuit, mechanical impact), because safety concerns remain a critical barrier for the introduction of any postlithium battery technology. Accelerating Rate Calorimeters (ARCs) are perfectly suited for these abuse tests. Fig. 7 shows the temperature vs. time curve during a HeatWaitSeek (HWS) test on a stack of two NVP/C//HC coin cells with an electrolyte composition of 1 M NaClO4 in EC:DMC:EMC (1:1:1) plus 2% FEC.
The plateaus in the temperature curve indicate the different stages of the reactions that finally lead to the thermal runaway. These can be seen more clearly in the two inserts. The first exothermic reaction represents the thermal decomposition of the solidelectrolyte interphase (SEI) layer. The second stage can be attributed to exothermic reactions between electrolyte and anode, which is no longer protected by the SEI layer. The third stage is the thermal runaway, which starts at 200 °C and reaches a maximum of 550 °C, before the cell is cooled down by pressurized air. By using a closed cell holder, which has an opening to a capillary connected to a pressure transducer the pressure increase by the gas that is formed during the different reactions can also be recorded. The maximum values for the temperature rate and the related pressure were 1.5 °C/min and 3.5 bar respectively. Recently the NVP/C based cells have been successfully upscaled to 5 cm × 5 cm pouch cells, which reached a maximum capacity of 18 mAh and a good cycling stability at C/5 charge/discharge rate with a remaining capacity of 88% after 300 cycles.
Thus, it has been demonstrated that the different types of calorimeters pave the way for mature and safe sodiumion batteries and other post lithium batteries.
How do the HELIOS battery packs guarantee sustainability, performance and improved safety?
HELIOS believes that a new concept of standardised, modular and scalable battery pack is possible, and that it will be efficient for use in a varied range of applications. Capitalising on advanced materials and topnotch modelling Big Data and Information and Communication Technologies (ICTs), the new battery pack is expected to combine improved autonomy and charging performance with enhanced safety standards and a minimum carbon footprint.
By bringing together the expertise of industry and academia, the HELIOS project aims at developing and integrating innovative materials, designs, technologies, and processes to create a new concept of a lighter, smarter, and more eco friendly hybrid battery pack. The hybridization concept consists of a combination of high energy (HE) and high power cells (HP). While the HE cells ensure a long driving range, the HP cells should enable fast charging and additional power for a short period if needed. The final battery pack should be modular and scalable for a wide range of electric vehicles used in urban electromobility services and should provide improved performance, energy density, safety, lifetime, and LCoS (Levelised Cost of Storage). The range encompasses midsize electric vehicles to electric buses. A Mitsubishi iMiEV at the Aarhus University and a Sileo S12 ebus from Bozankaya (see Figure 8) will be used as demonstrators for the performance and improvements of the HELIOS modular battery packs.
For this purpose, novel developments that integrate hardware and software solutions for the smart control of electrical and thermal management systems that exploit advanced materials, power electronics, sensors, and cuttingedge ICT, such as cloudbased Big Data Analysis, Artificial Intelligence, and IoT (Internet of Things) technologies running in the cloud are investigated and implemented.
These combined approaches allow us:
- To increase energy and power density;
- To enhance key characteristics like ultrahigh power charging;
- To improve safety;
- To improve Efleet control and health management strategies to extend lifetime;
- To create optimised EV charge and discharge procedures and predictive maintenance schedules;
- To monitor the SOC (State of Charge), SOH (State of Health), and carbon footprint for each battery pack throughout its entire life cycle, which allows an effective integrated supply chain for the manufacture, reuse, and recycling of Liion battery packs to be established;
- To show better LCA (life cycle assessment, new methodologies for recycling and metal recovery after EoL (end of life), and already a material selection with better overall LCA;
- To improve battery pack design and performance with reduced LCoS based on a circular economy approach where the modular battery packs can be easily reused in a range of second life applications prior to EoL recycling; and
- To assess the HELIOS solution effectiveness in different urban electromobility models such as car fleets and ebus fleets.
I lead the Work Package 4 – ‘Cell and battery pack testing and modelling’, whose main objectives are to expand and improve the stateoftheart within cell and battery modelling, state estimation, fault detection, prognostics, and health management by utilizing the available information from various sensing technologies and cell tests. It is also expected that the battery pack can be safer by being able to detect internal faults inside the battery before the faults propagate. The main scientific objective of the work performed at KIT, DTI, and ZSW is to determine the coupled electrochemical, thermal, and safety data of both the HE and HP cells that are required as input parameters for the modelling or as validation data for the simulation results. These data are acquired on the materials, cells, and pack level under both normal use and abuse testing scenarios for fresh and aged cells and are stored and aggregated in a data warehouse platform, that has been provided by DTI. The extensive cell testing will generate a large amount of data that will help to improve the different levels of modelling that will be developed in the third year in order to bring the HELIOS project to the road of success.

How does your work ensure lithium-ion batteries’ safety and high performance regarding cell ageing?
It is clear that safety issues have a major influence on consumers’ willingness to adopt the current Liion battery technology, because an uncontrollable temperature increase (socalled thermal runaway) can cause an ignition or even explosion of the battery with simultaneous release of toxic gases. Thus, thermal management and safety are of outmost importance for the electrification of transport and for stationary storage.
The abuse or safety testing has the main objective to identify all possible risk conditions to clearly define mitigation measures to be used in design, control and usage of the cells and packs. It is carried out using seven ARCs of different sizes – from cylindrical to large pouch or prismatic automotive format – by applying electrical, mechanical and thermal abuse scenarios and studying the heat, gas and pressure generation under these conditions. This allows quantifying critical parameters and their thresholds for safe cell operation. Moreover, the influence of ageing on the hazard potential can be quantitatively determined by performing the following three types of safety tests in the ARCs:
- Electrical abuse: External/ internal short circuit test, overcharge test, overdischarge test
- Mechanical abuse: Nail test
- Thermal abuse: HeatWaitSeek test, ramp heating test, thermal propagation test.
Selfheating, thermal stability and thermal runaway are characterized and the critical parameters and their thresholds for safe cell operation are determined. Another important issue are the venting gases, which can be collected during the abuse tests and analysed exsitu or measured operando using an online mass spectrometer. For the exsitu method a gas chromatography (GC) method with two detectors (mass spectrometry (MS) and thermal conductivity detector (TCD)) are available to detect and quantify the gases. Knowledge of the gas composition under the various cell abuse scenarios reveals the relevant decomposition paths and gives an idea, which protective measures are necessary, e.g. to delay or prevent the cell fire caused by ignition of vent gases or thermal runaway.
If it is not possible to stop a single cell from going into thermal runaway the propagation of the thermal runaway to the neighbouring cells, the socalled thermal propagation has to be prevented or at least extended by 510 min. The largest ARCs allow studying the thermal propagation on small pack level to develop and qualify suitable heat protection barriers, which give the passengers in an electric vehicle enough time to escape or to be rescued by the firefighters. This is currently a very hot topic because new regulations will come into force soon.
The cycle life can be studied by performing calendaric and cyclic ageing tests and characterising the cells at fixed time intervals with respect to changes in heat generation and temperature profile, which serves as a “fingerprint” for the stateofhealth (SOH). Comprehensive aging tests are carried out at the cell level for all cell sizes. At first, fresh cells are stored in temperature chambers at different temperatures and states of charge and then characterized at fixed time intervals in order to study the influence of this storage (calendar aging) on cell performance. At second, cells of the same type are aged at different charge / discharge rates or with different load profiles (full cycles, partial cycles, driving cycles stationary storage cycles (cyclic aging). Once the cells have reached their termination criterion, the reversible and the irreversible heat are measured using entropy measurement and electrochemical impedance spectroscopy (EIS). These data can be used for the parameterization of impedancebased electrical equivalent circuit models (EECMs) and ageing models. In addition, some cells are analysed post mortem, i.e. disassembled in an inert gas atmosphere and the changes in the chemical composition, the morphology and the structure of the electrodes are determined.
To summarize, battery calorimetry provides quantitative and system relevant data for temperature, heat and pressure development of materials and cells as a fast feedback for cell development and as input data for simulations.
References
- “Battery venting gas analysis using battery calorimeters & gas
chromatography”, Open Access Government 33, pages 384385. - “Correlation of electrolyte properties, gas formation and heat generation by electrochemicalcalorimetric methods”, Open Access Government 35, pages 382383.


