Knee pain and knee osteoarthritis degrade the quality of life of sufferers. Drawing from our clinical experience and research, we have identified ‘hidden lesions’ of knee osteoarthritis (knee OA) and scrutinized the role of medial abrasion phenomenon (MAP) in the pathogenesis of knee OA
Based on these findings, we have used arthroscopic medial release (AMR) and arthroscopic cartilage regeneration facilitating procedure (ACRFP) to eliminate MAP and related detrimental factors, reduce knee pain, improve quality of life, and even halt the deterioration process of knee OA for patients. We have employed these procedures at our practice to treat patients with knee OA over a span of about 20 years.
The encouraging clinical outcomes of our treatments are the foundation for our proposal to employ the Knee Health Promotion Option (KHPO) for the comprehensive treatment of knee OA.
1. Introduction
Although knee OA is a common progressive musculoskeletal condition and remains an immense public health concern worldwide, its pathophysiology is not yet well understood. Many physicians have traditionally described knee OA as a passive degenerative disease or “wear and tear” arthritis, but knee OA has more recently been described as a disease that involves the whole joint, characterized by cartilage erosion, subchondral bone remodeling, synovial inflammation, osteophytes formation, as well as degeneration of ligaments and menisci.
A lot of work has been put into searching for the etiology of knee OA and multiple hypotheses have been proposed, but still there is not a clear understanding of its natural course. Based on those hypotheses, traditional mainstream medicine has since developed a wide variety of treatments, such as pharmacological therapy, biologic intra-articular injection therapies (e.g., platelet-rich plasma, cell-based treatment options including bone marrow mesenchymal stem cells and autologous adipose stem cells), weight-shifting modalities (e.g., knee brace, wedge insole), or surgical procedures (e.g., arthroscopic debridement, microfracture, corrective osteotomy, autologous or allogeneic cartilage transplantation, and chondrocyte transplantation).
But all these treatments provide only symptomatic relief rather than preventative or regenerative outcomes, and may eventually lead the sufferers to total knee replacement. These treatments have failed to consistently and conclusively relieve pains and slow or arrest the process of chondral degradation. They failed because they didn’t know the cause of cartilage degradation. According to a qualitative interview study regarding patients’ and practitioners’ views of knee OA and its management [1], patients feel that their complaints are not taken seriously; they feel that practitioners act as technicians, paying more attention to the knee than to the patient; they consider that not enough time is spent on information and counseling; they have negative perceptions of drugs and they have a feeling of medical uncertainty about OA, which leads to less compliance with treatment and a switch to alternative medicine; patients believe that knee OA is an unavoidable illness associated with aging, that not much can be done to modify its evolution, that treatments are of little help, and that practitioners have not much to offer. Therefore, they express unrealistic fears about the impact of knee OA on daily and social life. On the other hand, the practitioners’ views differ from those of the patients. Physicians emphasize the difficulty in elaborating treatment strategies and the need for a tool to help them with treatment choices.
Starting from 2002, we conducted a series of studies to investigate medial abrasion phenomenon (MAP) as a cause of knee OA. We found medial plica-related MAP has a close correlation with knee OA. MAP would cause lifelong interplay between a pathologic medial plica and the facing medial femoral condyle, and therefore MAP plays a role in the pathogenesis of knee OA both physically and chemically. Consequently, we have developed the procedures of arthroscopic medial release (AMR) and arthroscopic cartilage regeneration facilitating procedure (ACRFP) for the treatment of knee OA.
Adding the removal of MAP to conventional surgical procedures such as osteotomy or arthroplasty could result in better outcomes for these procedures. The positive feedback from our patients encouraged us to develop a multidisciplinary treatment option in 2009, which we called the “knee health promotion option (KHPO)”, for the comprehensive management of knee OA based on the discovery of MAP as a cause of knee OA.
2. Medial abrasion phenomenon: a neglected cause of knee OA
In knees with medial plica and MAP, the repeated abrasion and impingement between medial plica and medial femoral condyle will elicit both physical and chemical damage to both the adjacent cartilage and the medial plica itself. This MAP may have played a role in the pathogenesis of knee OA.
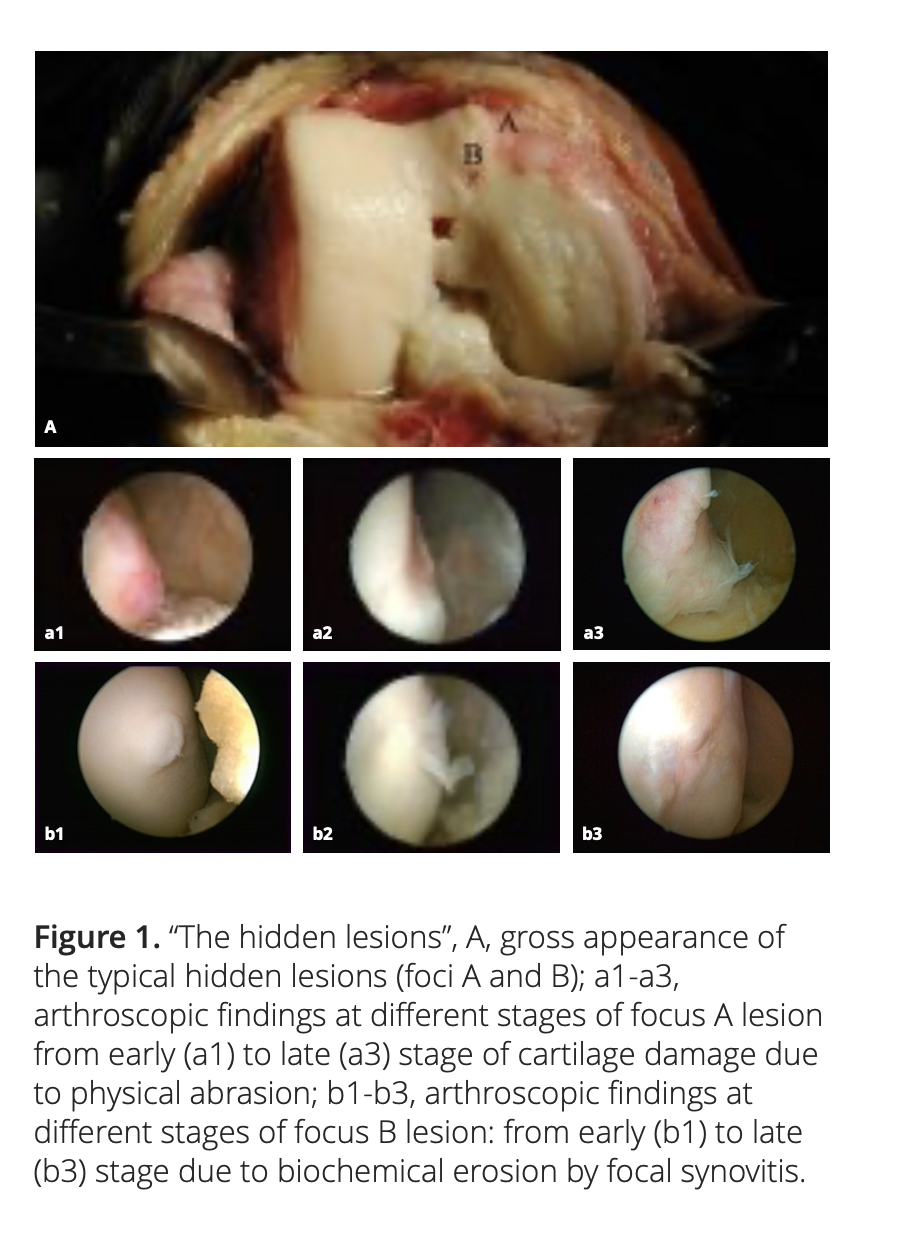
2.1 Medial plica and the hidden lesions
Medial plica is a fold in the synovium that represents an embryologic remnant in the development of the knee’s synovial cavity. Our clinical experience shows that medial plica is present in about 95% of the patients with chronic knee pain, and knee OA is their most common clinical diagnosis. The repeated abrasion between medial plica and the facing medial femoral condyle in daily activities will increase the severity of the pathologic change of the medial plica and will simultaneously decrease its size due to wear.
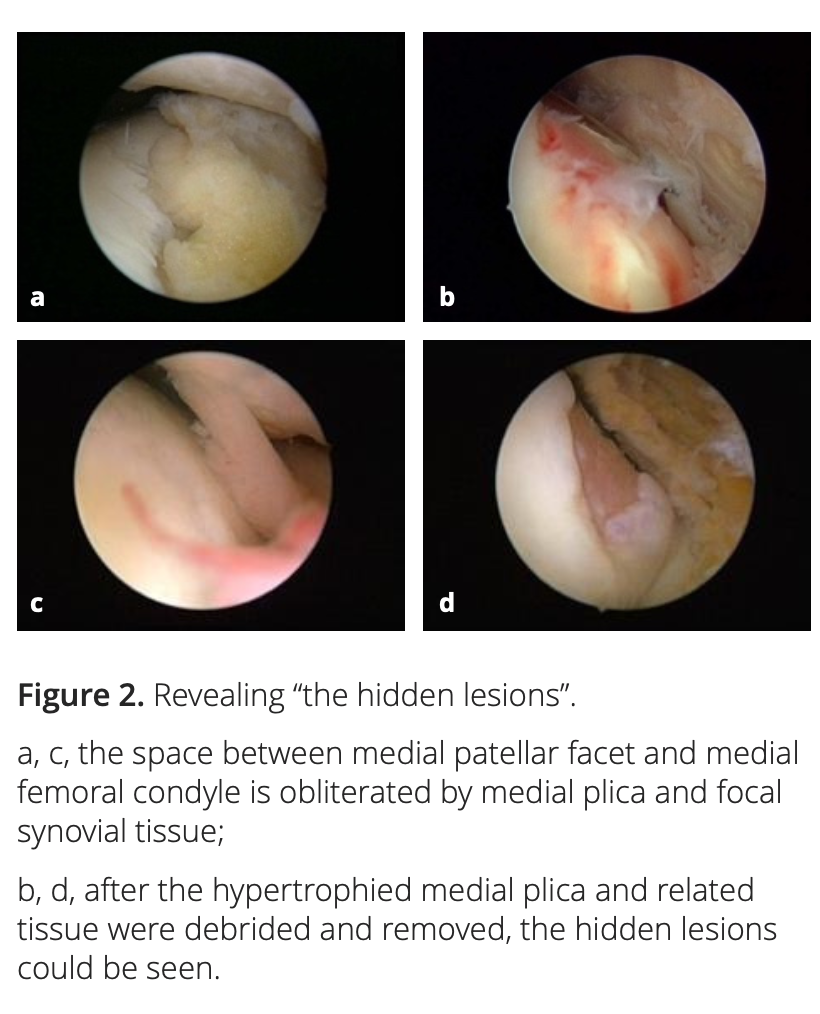
After adequate synovectomy and releasing of the inferomedial area of the patella and medial gutter, we could see two distinct foci of cartilaginous lesions on the edge and the anterior part of the medial femoral condyle (Figure 1). We call them ‘‘hidden lesions’’ because on most occasions, due to either synovitis or the tightness of the joint space, these lesions were easily overlooked during routine arthroscopic examination. Only after adequate medial release did they become visible (Figure 2). We have found that the severity of degeneration in each individual location and in the whole knee are negatively correlated with the size and positively correlated with the severity of the medial plica [2].
2.2 MAP as a cause of knee OA
Thought to be multifactorial, the causes and pathogenesis of knee OA are not fully understood. During the process of OA, changing cartilage composition and loss of cartilage integrity increase the susceptibility to disruption of the cartilage [3]. Degenerative shifts in the cartilage lead to an increased production of extracellular matrix fragments, which promote the release of pro-inflammatory cytokines, like interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α [4]. Once secreted, these cytokines can modulate chondrocytes and adjacent synoviocytes metabolism, inducing the secretion of proteolytic enzymes, such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), which in turn aids in cartilage degradation and fragmentation. Elevated ECM fragments additionally stimulate the release of pro-inflammatory mediators and matrix degradation products, forming a vicious circle [5, 6]. However, it is still unknown what triggers this vicious circle.
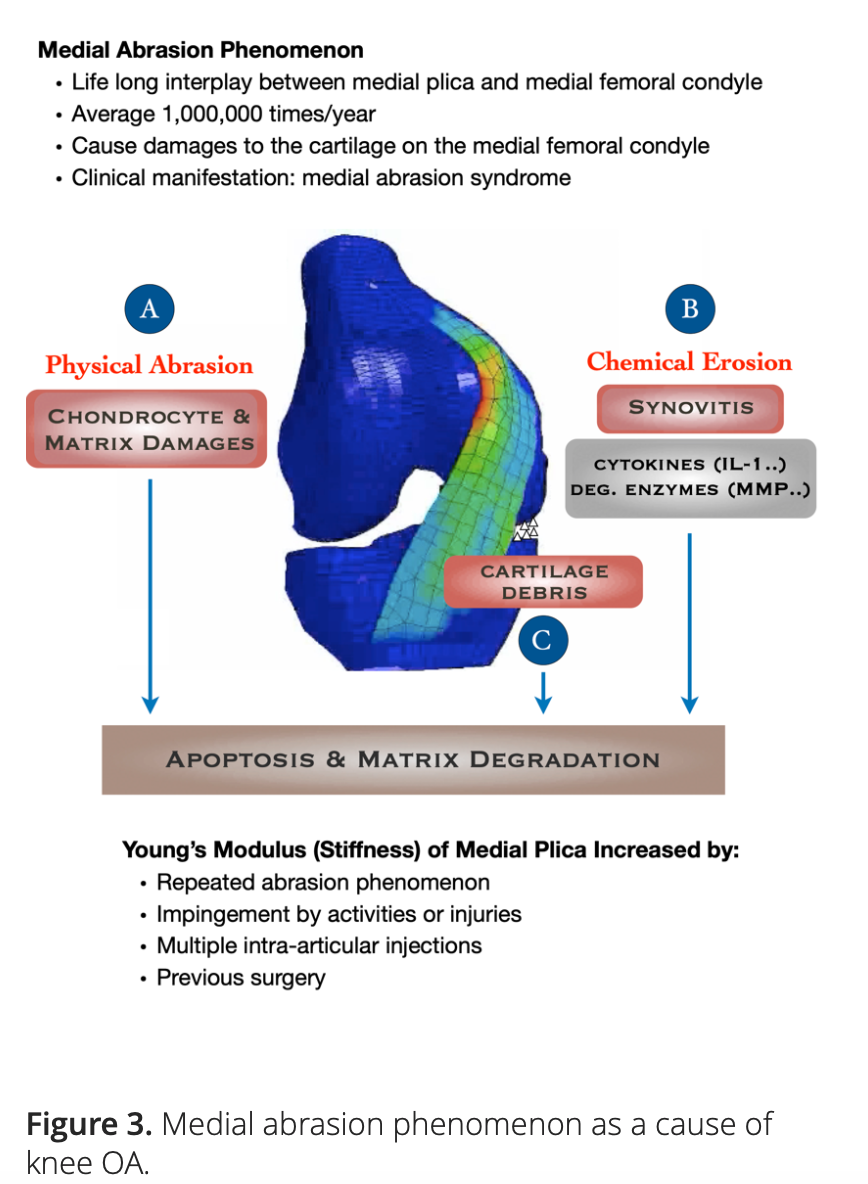
Our hypothesis points to the role of MAP as an initiating factor in the pathogenesis of knee OA. There is growing evidence suggesting that both the physical and biochemical effects elicited by the repeated interplay between medial plica and the facing medial femoral condyle may contribute to the development and progression of knee OA (Figure 3).
2.2.1 The construction of a three-dimensional dynamic finite element model
We conducted an experimental study on the tensile strength of the medial plica from 50 knees. Using high-precision micro-force tensile tests, we found that Young’s modulus of the plica ranged from 10 to 110MPa and had a positive correlation with the patient’s age [7]. In another selected 30 knees with different sizes of medial plica, we located the inner margins of the plicae by inserting needles percutaneously under direct vision during arthroscopic examination. The topographic changes of the margins of these plicae during knee motion were recorded with fluoroscopy and analyzed [8].
Based on these two pilot studies, we constructed a three-dimensional dynamic finite element model, which we used to investigate the magnitudes of the cyclic pressures acting on the cartilage of the medial femoral condyles by different types of medial plicae with various Young’s moduli. The contact pressures on the cartilage of the medial femoral condyles were found to be positively correlated with Young’s moduli of the medial plicae. When Young’s moduli were set greater than 60 MPa to simulate the medial plicae of middle and old aged patients whose knees were bent beyond 50°, all types of medial plicae would elicit contact pressures high enough to cause apoptosis of the chondrocytes on the cartilage of the medial femoral condyles [9].
2.2.2 Histomorphology study
We have investigated the gross appearance and histological features of the medial plicae removed from 48 consecutive patients who had received total knee replacement for severe medial compartment OA of their knees [10]. The prevalence of the MAP was 100%. Histologically, most of the advanced pathologic manifestations were found at the middle and distal portion of the medial plica that might abrade the articular cartilage of the medial femoral condyle. Noticeable cartilaginous lesions were found on the facing medial femoral condyle in all knees. The histomorphology findings of the medial plica imply the close interplay between this structure and medial femoral condyle that might play a role in the pathogenesis of medial compartment OA of the knee.
Furthermore, a small branch of skeletal muscle originating from articularis genu inserting into the proximal synovial stroma of the medial plica was found in all knees. We found that the synovial fold of the distal part of the medial plica has a close correlation with the gracilis tendon sheath. A longitudinal case study analysis [11] suggested that abnormal gait patterns may lead to the development of knee OA. It has been pointed out in several studies [12-14] that an increased knee adduction and internal rotation moment during the stance phase of gait is associated with knee OA. It was also detected that among individuals with mild radiographic knee OA, those who are symptomatic have significantly higher medial compartment loads than those who are asymptomatic [15].
The linkage between medial plica and the medial muscle group of the thigh found in this study might help explain these literature findings that revealed the relationship between gaits and medial compartment OA knee. During the walking cycle, the irritation of the inflamed medial plicae elicited by the abrasion phenomenon with the medial femoral condyle might evoke reflex contracture of the medial muscle group, including vastus intermedialis and gracilis and, therefore, increase the adduction and internal rotation moment and the medial compartment load of the knee. This correlation could further be proven by subjective improvement of gait pattern claimed by some of our OA knee patients who have received arthroscopic resection of the inflamed medial plica [16].
2.2.3 Biochemical analysis
In our 2007 study, the inflamed medial plicae, pannus-like tissues, and adjacent cartilage on the medial femoral condyle of 24 knees with medial compartment OA who had undergone AMR to remove their medial plica were obtained for investigation. The synovial membrane of the lateral gutter of each knee was sampled as a control. Immunohistochemistry showed that matrix metalloproteinase (MMP)-3 was highly expressed in pannus-like tissue and the plica. Western blotting of culture supernatants showed that interleukin (IL)-1β treatment would induce cells isolated from pannus tissue or the plica to release MMP-3.
Furthermore, reverse transcriptase polymerase chain reaction and real-time polymerase chain reaction analysis showed that MMP-3 mRNA levels were increased after IL-1β treatment of the cultured cells. MMP-3 and IL-1β mRNAs were expressed in the plica and pannus-like tissue, with MMP-3 mRNA being expressed at significantly higher levels in the plica than in normal synovial membrane and highly expressed in the plica at different stages in OA knee patients [17].
In another study, we examined the expression of MMPs, tissue inhibitors of metalloproteinases (TIMPs), IL-1β, and tumor necrosis factor (TNF)-α in the medial plica and pannus-like tissue in the knees of OA knee patients. Fifteen specimens of medial plica and pannus-like tissue were obtained from 15 patients with Kellgren and Lawrence grade II (stage II) medial compartment OA who had undergone AMR and another 18 specimens were obtained from 18 patients with grade IV medial compartment OA who had undergone total knee arthroplasty.
The MMP-2, MMP-3, MMP-9, IL-1β, and TNF-α mRNA, and protein levels measured, respectively, by quantitative real-time PCT and Quantibody human MMP arrays, were highly expressed in extracts of medial plica and pannus-like tissue from stage IV knee joints. Immunohistochemical staining also demonstrated high expression of MMP-2, MMP-3, and MMP-9 in plica and pannus-like tissue of stage IV OA knees but not in normal cartilage. Some TIMP/MMP ratios decrease significantly in both medial plica and pannus-like tissue as the disease progresses from stage II to stage IV. Furthermore, the migration of cells from the pannus-like tissue was enhanced by IL-1β, while plica cell migration was enhanced by TNF-α [18].
These biochemical analyses demonstrated 1) that medial plica and the pannus-like tissue produced by MAP on the facing femoral condyle may have played a role in the process of cartilage degradation in osteoarthritic knees by producing extracellular matrix degradation enzymes during inflammation and 2) that the imbalance between TIMP and MMP levels in this tissue increases protease activity in the medial compartment and this may also have contributed to cartilage breakdown and progression of osteoarthritis.
We also collected 14 sets of synovial fluid from the medial and lateral compartments of the knees of 14 patients who had Kellgren and Lawrence grade IV medial compartment knee OA for which they had received unicompartmental arthroplasty. The total protein, TNF-α, IL-1β and MMP-3 concentrations of the synovial fluid obtained from both medial and lateral compartments of these knees were measured. Fibrotic or hypertrophied medial plica demonstrating abrasion phenomenon with the facing medial femoral condyle was found in all these knees. Every set of the samples revealed significantly higher total protein, TNF-α, IL-1β and MMP-3 concentrations in the medial compartment. These findings have further solidified the role of MAP in the pathogenesis or progression of knee OA [19].


