Current IBD treatments leave many battling uncomfortable symptoms. The bodies natural endocannabanoid system (ECS) is linked to gut health. By targeting the system, researchers believe they will be able to develop novel treatments that tackle inflammation, diarrhea and lingering discomfort. By focusing on this, researchers believe they will be able to develop treatments that tackle both inflammation and lingering discomfort
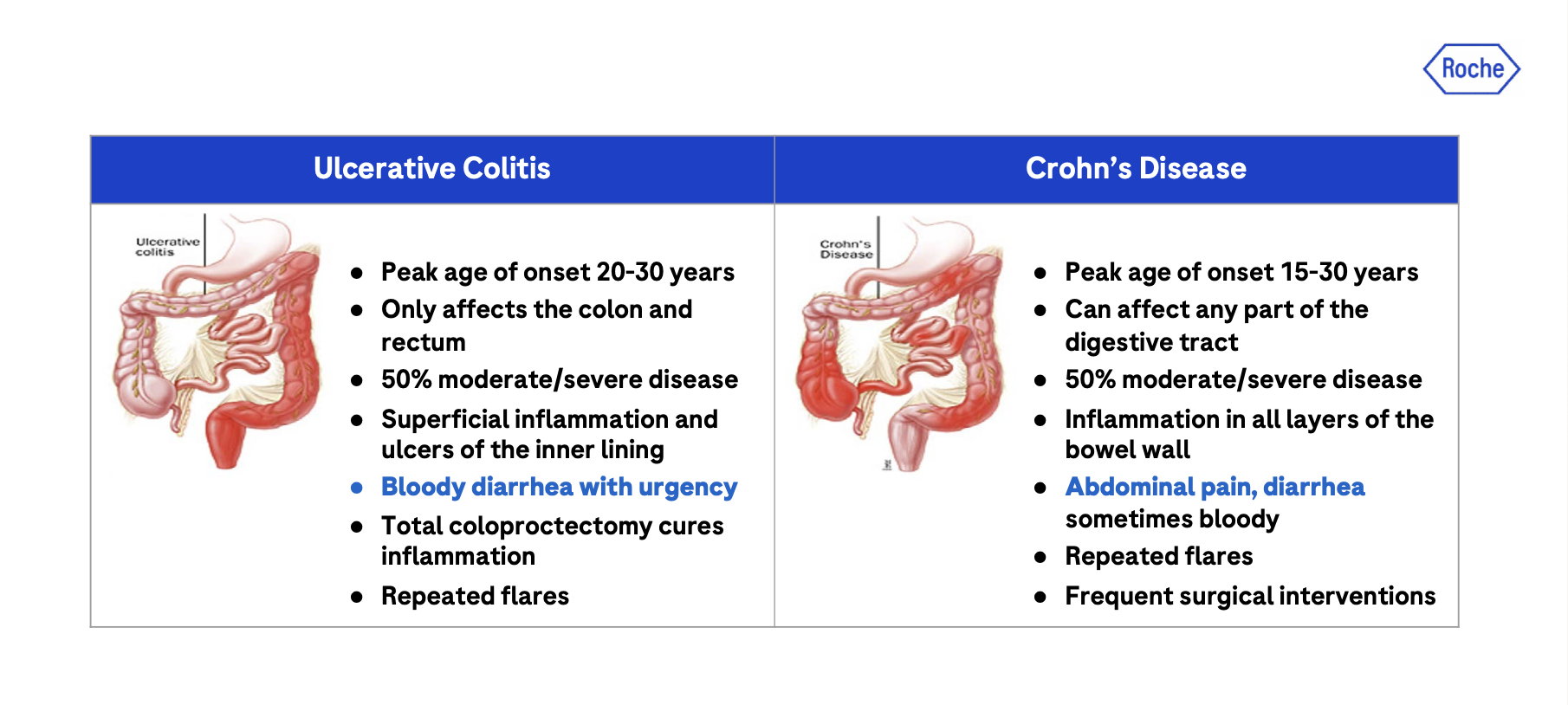
Inflammatory Bowel Diseases (IBD), i.e. Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic debilitating diseases of the intestinal tract that remain inadequately controlled with Standard of Care (SoC) and new therapies (Figure 1). The etiology of the diseases and the precipitator of the relapsing intestinal inflammation are unknown and include genetic factors (e.g., de Lange et al., 2017), and still unidentified environmental and likely microbial factors (e.g., Borowitz, 2022).
Current treatment aims to bring the intestinal mucosal inflammation under control. However, the correlation between improvement of the mucosal inflammatory burden as detected by C-reactive protein (CRP) or endoscopy, and the resolution of symptoms of IBD is very poor (Peyrin-Biroulet et al., 2014, Rutgeerts et al., 2006). Abdominal pain is the most prevalent persistent symptom in patients with IBD that have resolved mucosal inflammation, followed by diarrhea, fatigue and other symptoms. Up to 40% of patients with IBD in endoscopic remission have persistent symptoms (Halpin and Ford, 2012; Lönnfors et al., 2014; Schirbel et al., 2010). Persistent symptoms also lead to a high societal burden, including loss of school or working hours (Cross et al., 2022; Youseff et al., 2022). Although persistent symptoms of IBD are a high burden to patients and to society, there is no targeted therapy available nor, to our knowledge, in development.
The poor correlation between the burden of superficial mucosal inflammation detected by endoscopy and symptoms such as abdominal pain, diarrhea, bloating, and fatigue, suggests that these manifestations of IBD may have a separate biological cause. The symptoms of IBD resemble the symptoms of irritable bowel syndrome (IBS). Severe intestinal infections often lead to post-infectious IBS and postinfectious dyspepsia (Wang and Fang, 2021) which are associates with a long-lasting sensitization of neuronel signaling in the gut (Balemans et al., 2017). This suggests that inflammatory processes are underlying the symptoms in IBS and IBD. Indeed, studies in IBS and dyspepsia patients imply macrophages and activated mast cells in proximity of enteric nerves as the cause of abdominal pain (Barbara et al., 2004; Li et al., 2010).
We present in this paper that the endocannabinoid system may play a crucial role in IBD.
The endocannabinoid system (ECS)
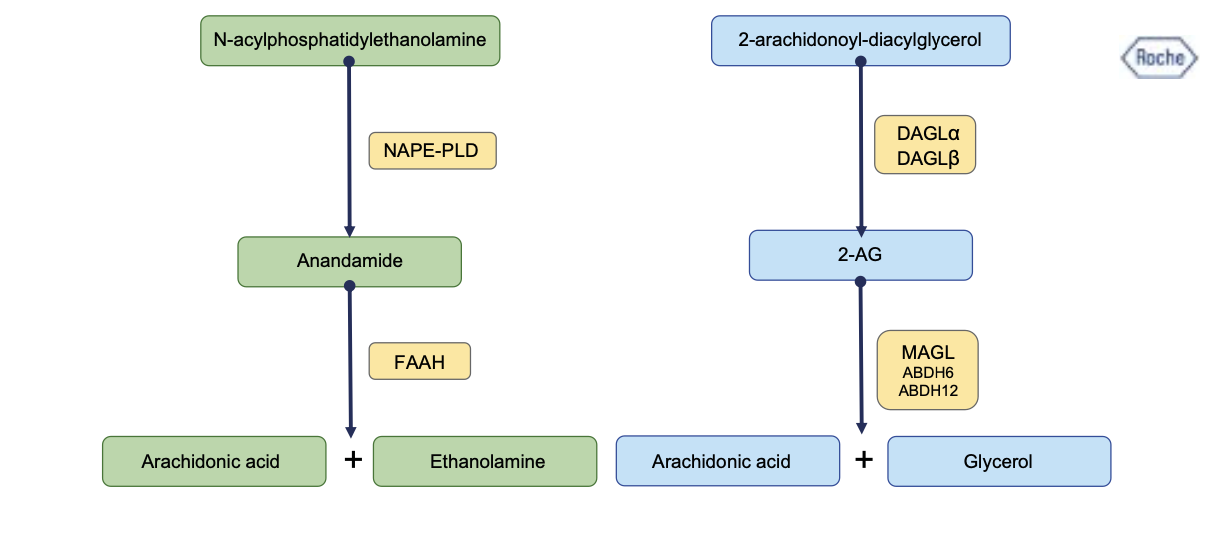
Endocannabinoids are lipid-based signaling molecules that are produced and released on demand and modulate visceral physiology and immunity (Cani et al., 2016; Turcotte et al., 2015). The 2 main endocannabinoids are N-arachidonoylethanolamine (AEA, or synonymous anadamide) and 2-arachidonoylglycerol (2-AG) (Maccarrone et al., 2023).
The endocannabinoid system (ECS) comprises the endocannabinoids, the enzymes involved in synthesis and degradation of endocannabinoids and their receptors (Figure 2).
The enzymes involved in endocannabinoid biosynthesis are DAGLα, DAGLβ and NAPE- PLD.
- The enzymes involved in endocannabinoid degradation are fatty acid amine hydrolase (FAAH) but also by cyclooxygenase-2 (COX-2) and lipoxygenases (LOXs), and monoacylglycerol lipase (MAGL, also termed monoglyceride lipase). AEA is mainly degraded by FAAH but also by cyclooxygenase-2 (COX-2) and lipoxygenases (LOXs). 2-AG is mainly degraded by MAGL and sometimes by COX-2, LOXs and minor enzymes such as α/β hydrolase-6 (ABHD6) and α/β hydrolase-12 (ABHD12) (Maccarrone et al., 2023).
- The cannabinoid receptors 1 and 2 (CB1R and CB2R, respectively) are the receptors for AEA and 2-AG.
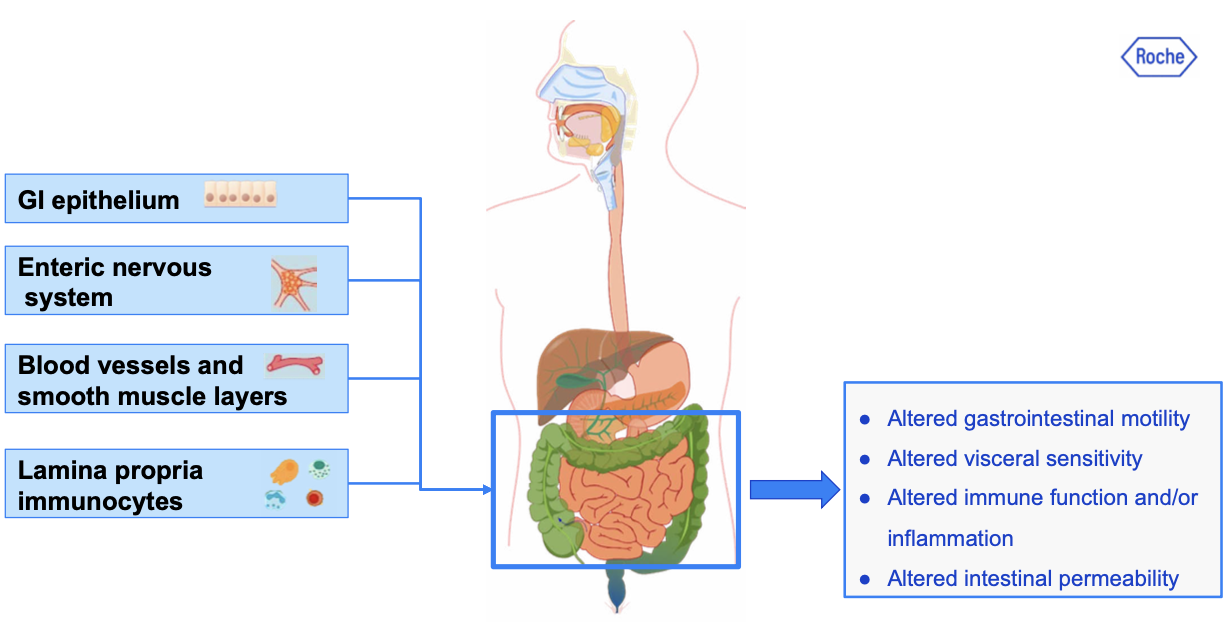
Endocannabinoids are involved in diverse gastrointestinal functions including gastric secretion and gastroprotection, nausea and emesis, gut motility, mucosal permeability, visceral sensation, intestinal inflammation, and cell proliferation (see Figure 3) (Camilleri and Zheng, 2023; Blaising and Smith, 2024).
One of the major endo cannabinoids in the gut is 2-arachidonoyl glycerol (2-AG). 2-AG is synthesized through the lipolysis of phospholipid precursors and activates the G-protein-coupled cannabinoid receptors CB1R and CB2R.
- CB1R is highly expressed on enteric neurons in the gut (Blaising and Smith, 2024). Its activation on enteric nerves induces a decrease of neurotransmitter release into the synapse and therefore a depression of excitatory neurons in the ENS (Cuddihey et al., 2022). This leads to biological effects such as pain (hypersensitivity) and diarrhea or constipation. CB1R is also expressed in the gastrointestinal epithelium and can modulate intestinal ion transport and intestinal permeability (Camilleri and Zheng, 2023).
- CB2R is expressed predominantly on immune cells such as macrophages and mast cells which mediate the anti-inflammatory effects of endocannabinoids in the GI tract (Blaising and Smith, 2024). Activation of CB2R inhibits the release of immune mediators or neurotransmitters.
The potential of Targeting the endocannabinoid system (ECS) to treat IBD
Several studies in disease models of colitis have demonstrated the effects of cannabinoids in bowel inflammation, visceral pain and motility (Cuddihey et al., 2022).
Targeting CB1R and CB2R
Activation of CB1R and CB2R reduced hypersensitivity and pain induced by experimental colitis (Camilleri and Zheng, 2023.
It has been also demonstrated that CB1R and CB2R knockout mice are more susceptible to chemically induced colitis (Massa and al., 2004; Engel et al., 2010).
CB1R is particularly involved in visceral pain. CB1R mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents (Brusberg et al., 2009). CB1R regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon (Sledent et al., 1999; Sibaev et al., 2009).
Selective CB2R agonists demonstrated efficacy in colitis-induced acute and chronic visceral hypersensitivity in rodents (Lindstrom et al., 2019; Castro et al., 2022).
Immunohistochemical studies on IBD patients have shown that the expression of CB2R is amplified during inflammatory flares, thus suggesting that they are a potential therapeutic target (Marquez et al., 2009).
The effect of targeting CB2R on abdominal pain in patients with Crohn’s disease has been explored in a open-label Phase 2a study of 25 mg TID or 100 mg TID olorinab, a CB2R-selective agonist (Yacyshyn et al., 2020). The absence of a placebo control group limits the conclusions from this study. Nevertheless, it is interesting that a non-dose-dependent reduction of the abdominal pain score was observed already on Day 1; the pain improvement was statistically significant at the predefined endpoints in Week 4 and Week 8.
Targeting the enzymes of the ECS
Another therapeutic strategy is to target enzymes that increase or decrease the endogenous CB receptor agonists. Inhibiting the enzymes responsible for endocannabinoid synthesis would reduce the concentration of endogenous CB1R and CB2R ligands.
We discussed in the previous sections that activation of these two receptors showed broad potential for improving IBD in terms of inflammation and abdominal pain.
Inhibiting the enzyme responsible for ECS synthesis would go against these beneficial effect driven via CB1R and CB2R and therefore, might not have a broad therapeutic benefit. DAGL inhibitors orlistat and OMDM-188 accelerated colonic transit in mice, proving the concept that inhibition of endocannabinoid synthesis may potentially provide a novel approach for relieving constipation (Bashashati et al., 2015). Nevertheless, constipation is not a major symptom of IBD and this approach would be applicable for IBS-C.
Inhibitors of endocannabinoid degradation decrease intestinal motility, peristalsis, and colonic propulsion in rodents, primarily in a CB1R-dependent manner.
FAAH deletion reduced pain sensation, and when exogenous AEA was administered FAAH- 1-deficient mice exhibited intense hypomotility, antinociception, catalepsy, and hypothermia in a CB1R-dependent manner (Maccarone et al., 2023). FAAH-deficient mice as well as inhibitors of anandamide reuptake or enzymatic hydrolysis showed protection against colonic inflammation. Anandamide levels and expression of cannabinoid receptors were increased in the inflamed colon (Camilleri and Zheng, 2023). Several FAAH inhibitors (PF-3845, VDM11, AA-5-HT) demonstrated efficacy in rodent colitis models by decreasing colonic inflammation (Alhouayek and Muccioli, 2012; Salaga et al., 2014).
As described in the previous section, MAGL is the main enzyme responsible for the inactivation of 2-AG. Mice with a genetic deletion of MAGL (Mgll knockout mice) exhibit elevated 2-AG levels, complete insensitivity to CB1R agonist-mediated inhibition of whole gut transit and ileal contractility suggesting local desensitization of CB1R, and accelerated colonic propulsion and hypersensitivity to μ receptor agonist-mediated inhibition of colonic motility (Taschler et al., 2015).
Elevating levels of 2-AG using a pharmacologic MAGL inhibitor attenuated murine TNBS colitis and significantly improved intestinal barrier function (Alhouayek et al., 2011).
Various pharmacological strategies can be used to modulate endocannabinoid levels and improve IBD by inhibition of endocannabinoid degradation (FAAH and MAGL inhibitors), or direct activation of CB1R and CB2R through synthetic CB1/2R agonists (Maccarone et al., 2023).
Conclusions and perspectives
As discussed in this article, the ECS constitutes an attractive therapeutic target for the treatment of GI inflammatory disease and associated symptoms. Specifically, effects on the CB2R axis may be an anti-inflammatory treatment modality distinct from all current modes of actions with potential as monotherapy and also as a valuable combination partner with current Standard of Care. Effects on the CB1R axis, on the other hand, are expected to be a novel and first-in-disease treatment for symptoms of IBD, including persistent symptoms described to be present in 30% to 40% of all IBD patients.
In regards to safety, cannabinoids may cause CB1R-mediated adverse events such as tachycardia, nausea, psychosis mania and suicidal behavior. To mitigate the safety risks peripheral restriction and/or selectivity for specific target tissues may promote beneficial therapeutic responses while avoiding central cannabimimetic side effects.
Most of the more recent marketed drugs for IBD are anti-inflammatory, targeting T cell migration (anti-integrin and S1P), T cell differentiation (anti-IL-23) and cytokine signaling (Jak inhibitor). However, there remains significant unmet medical need as most existing treatments only achieve maintenance of placebo-adjusted remission rates of less than 25% at 12 months (Feagan, 2013; Feagan, 2016; Sandborn, 2017; Sandborn, 2021). Furthermore, these therapies can also lose effectiveness over time (Roda, 2016). Therapeutically modulating the ECS in combination with standard-of-care established therapies may offer an innovative approach to treat residual inflammation and symptoms of IBD not addressed by the standard of care.


