Here, we discover that FebriDx® rapid point of care triage can help prevent the spread of infection and improve patient streaming
The COVID-19 pandemic continues to place additional workload on all healthcare settings, particularly Emergency Departments (ED). The long turnaround time of laboratory RT-PCR SARS-CoV-2 testing results in delayed diagnosis and slows patient isolation decisions which places patients at risk for cross infection. Rapid antigen tests are available for SARS-CoV-2; however, whilst they are rapid and easy to use, their performance in a real-World setting has been shown to be low compared to RT-PCR (Scohy).
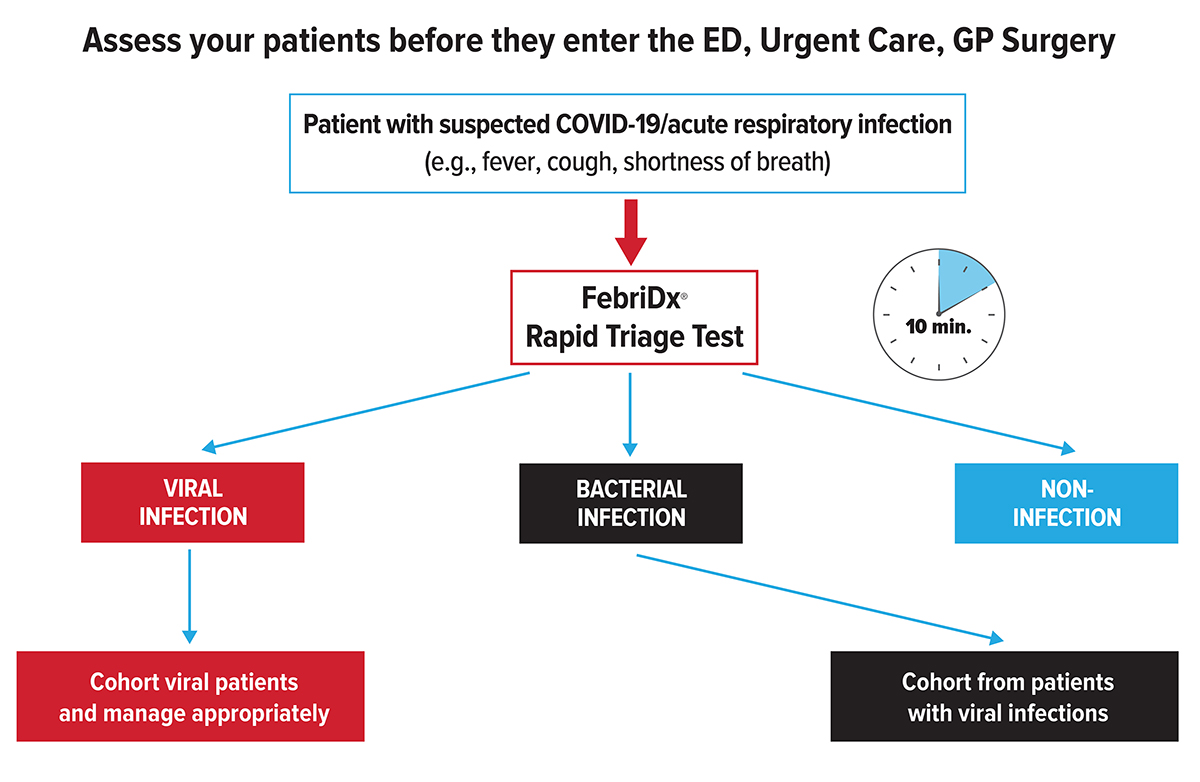
The significant influx of patients caused by COVID-19 has overwhelmed healthcare systems, underscoring the need to rapidly triage patients at the point of entry. Clinicians need to be able to quickly and accurately cohort suspected positive COVID-19 patients away from negative patients to prevent the spread of infection and maximise the limited availability of single occupancy isolation units.
Hospitals ED’s have set up front end processes to try and separate suspected COVID-19 from non-COVID-19 patients, however, the long turnaround of laboratory RT-PCR testing together with instrument throughput challenges precludes results being available at the point-of-care. This has highlighted the need for accurate tests that can rapidly triage and cohort potential COVID-19 patients away from negative patients improving patient management, patient streaming and workflow.
FebriDx a rapid patient Triage test: Compelling evidence from UK hospital studies
Utility of the FebriDx point-of-care test for rapid triage and identification of possible coronavirus disease 2019 (COVID-19) Karim et al
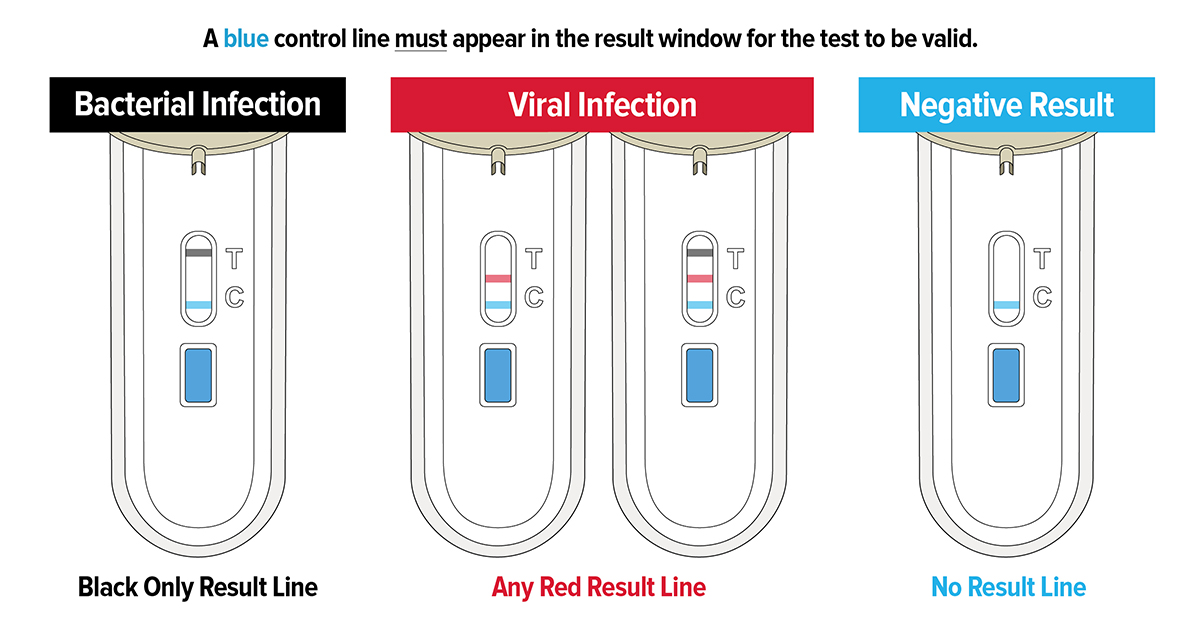
Karim et al evaluated the utility of FebriDx® on 47 patients suspected to have COVID-19 to reduce time to presumed diagnosis and allow for appropriate isolation. Using the European Centre for Disease Prevention and Control (ECDC) COVID-19 case definitions, 34 patients were confirmed to have a final diagnosis of COVID-19. FebriDx® detected 34/34 (100%) of COVID-19 infections by MxA specific viral biomarker whilst the initial SARS-CoV-2 PCR test identified 29/34 (85.3%). FebriDx® provided results in 10 minutes whilst RT-PCR had a 48-hour turnaround time. The use of FebriDx® as an initial triage test may have prevented COVID-19 negative patients being exposed to COVID-19 positive patients caused by the delay in molecular test results.
During a pandemic it is also imperative to ensure that patients with other infections (e.g. bacterial) are not missed, 8/47 of the patients (negative for COVID-19) were shown to have a bacterial infection. FebriDx® detected 8/8 bacterial infections (100% sensitive; 92.5% specific). Karim et al concluded that “FebriDx testing can improve time to initial triage and isolation and could be deployed as part of a reliable triage strategy for identifying symptomatic cases as possible COVID-19 in the pandemic”.
Diagnostic accuracy of the FebriDx host response point-of-care test in patients hospitalised with suspected COVID-19 Clark et al.
Clark et al prospectively evaluated FebriDx® for the detection of COVID-19 in hospitalised patients during the first wave of the pandemic to improve patient flow and reduce the spread of infection. FebriDx® results were available within 10 minutes compared to ~24 hours for laboratory PCR testing. FebriDx® was shown to be 93% (110/118) sensitive and 86% (112/130) specific for detection of COVID-19 infections by MxA biomarker compared to PCR. Several patients with FebriDx® viral positive results (negative by PCR) had classical radiological features of COVID-19, thus were likely true positives and false-positive PCR results.
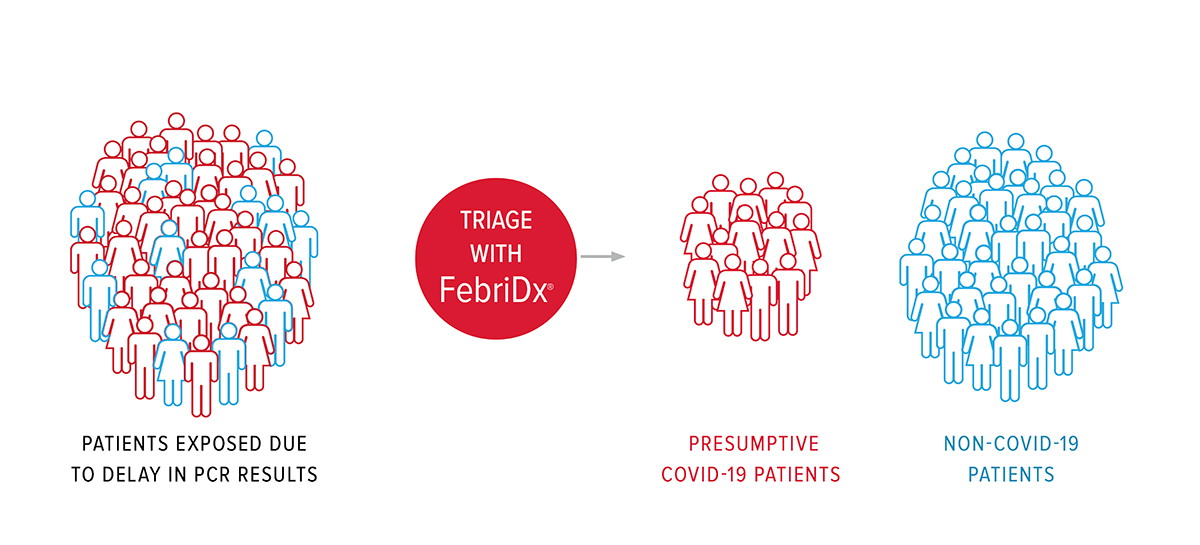
Due to the high sensitivity and negative predictive value (NPV), FebriDx® viral negative patients can be rapidly cohorted in non-COVID-19 areas, allowing FebriDx® viral positive patients to be immediately isolated whilst awaiting confirmatory testing. FebriDx® NPV was calculated to be 99% at 10-20% COVID-19 prevalence, concluding that a negative FebriDx® is a useful rule-out test.
Clark et al concluded that FebriDx® was shown to be highly accurate in the rapid identification of COVID-19 infections and could be rapidly deployed as a front door triage tool in hospitals and urgent care centres to overcome current issues of delayed diagnosis from PCR testing.
FebriDx®: A rapid patient triage test
FebriDx® allows clinicians to:
Rapidly identify presumptive COVID-19 patients;
Facilitate immediate patient isolation;
Prevent the spread of infection;
Ensure patients with bacterial infections are not missed;
Improve patient streaming and reduce healthcare costs.
Save time and money with FebriDx® triage
Rapid triaging with FebriDx® can prevent the spread of infection. Direct medical costs for a single COVID-19 infection is estimated to be $3,045 (Bartsch). FebriDx® can also save healthcare costs in terms of diagnostic stewardship and reduction of unnecessary PCR confirmatory tests from patients who are viral negative.
FebriDx® Technology
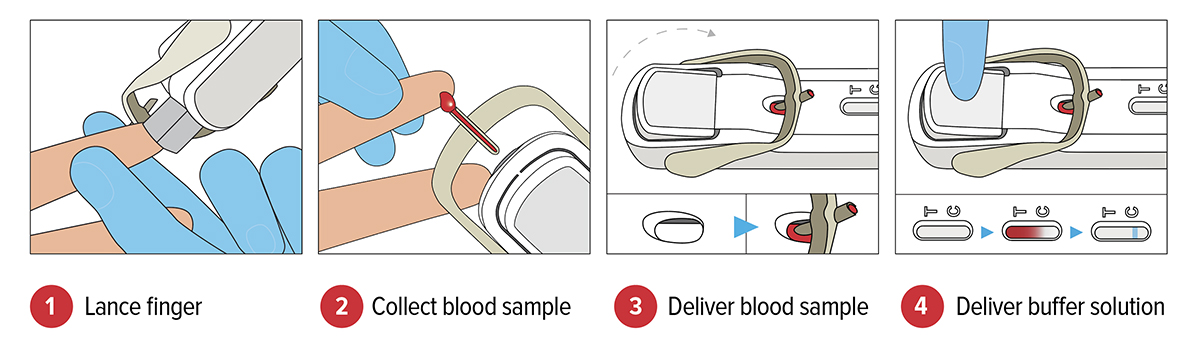
FebriDx® is the first and only rapid POC test to differentiate a viral from bacterial acute respiratory infection (ARI) through the simultaneous detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from a fingerstick blood sample in just 10 minutes. FebriDx® is an ‘all in one’ style portable POC test which incorporates an onboard fingerstick sample collection.
CRP is a non-specific acute phase inflammatory protein that is elevated in both viral and bacterial infections whilst MxA becomes elevated only in an acute viral infection (Simon) including COVID-19 and not in bacterial infection. MxA is an intracellular blood protein that is stimulated by interferon (IFN) alpha/beta cells. IFN cells are induced by viruses and form an essential part of the immune system’s defence against viral infections. MxA has a fast induction time of on to two hours and a long half-life of 2.3 days, making it an ideal biomarker for acute viral infections (Nakabayashi).
Conclusion
The ability to rapidly triage patients at the point of contact, when clinical management decisions are being made, including the need for isolation is a critical component of the national pandemic response. FebriDx® is the first and only rapid test to differentiate a viral from bacterial acute respiratory infection and is being used in hospitals and other healthcare settings to rapidly triage symptomatic patients with ARI/suspected COVID-19.
FebriDx® can enable effective triaging, so viral negative patients can be rapidly cohorted in non-COVID-19 areas, allowing viral positive patients to be immediately isolated while awaiting confirmatory testing to prevent the spread of infection. UK National Institute for Health & Care Excellence (NICE) recently included an updated FebriDx MedTech Innovation Brief on the specific COVID-19 section of the NICE website which is reserved for technologies which can help in the Pandemic.
FebriDx® has been demonstrated to be highly accurate at rapidly detecting COVID-19 infections through the MxA specific viral biomarker including infections missed by RT-PCR testing; and can be rapidly deployed as a front door triage tool in hospitals, Primary Care and Care Homes to overcome current issues of delayed diagnosis from PCR testing.
References
Devine, C., Covid-19 testing: A spike in demand, a delay on results, in CNN. July 7, 2020. Accessed on July 22, 2020. Available from: https://www.cnn.com/2020/07/07/politics/coronavirus-testing-delays-invs/index.html.
Scohy, Anaïs, Ahalieyah Anantharajah, Monique Bodéus, Benoît Kabamba-Mukadi, Alexia Verroken, and Hector Rodriguez-Villalobos. 2020. ‘Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis’, Journal of Clinical Virology: 104455.
Karim, Nawazish, Muhammad Zubair Ashraf, Muhammad Naeem, Tahir Anwar, Hnin Aung, Srikumar Mallik, Eleni Avraam, Sidra Kiran, Sareesh Bandapaati, and Faisal Khan. 2020. ‘Utility of FebriDx® in early identification of possible COVID19 infection’.
The European Centers for Disease Prevention and Control (ECDC). “Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020 “ Accessed on April 21, 2020. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition.
Clark, Tristan W, Nathan J Brendish, Stephen Poole, Vasanth V Naidu, Christopher Mansbridge, Nicholas Norton, Helen Wheeler, Laura Presland, and Sean Ewings. 2020. ‘Diagnostic accuracy of the FebriDx® host response point-of-care test in patients hospitalised with suspected COVID-19’, Journal of Infection.
Bartsch SM, Feruson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020;39(6):927–935. https://doi.org/10.1377/hlthaff.2020.00426
Simon, A., J. Fah, O. Haller, and P. Staeheli. 1991. ‘Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines’, Journal of virology, 65: 968-71.
Nakabayashi, Motokazu, Yuichi Adachi, Toshiko Itazawa, Yoshie Okabe, Hirokazu Kanegane, Mizuho Kawamura, Akihito Tomita, and Toshio Miyawaki. 2006. ‘MxA-based recognition of viral illness in febrile children by a whole blood assay’, Pediatric research, 60: 770-74.
https://www.nice.org.uk/covid-19
FebriDx® is not currently available in the United States.
Please note: This is a commercial profile