Despite the rising incidence of Central Nervous System diseases worldwide, efficient treatment options for neurological disorders remain scarce. Treatment failures are principally associated with the inability of drugs to pass the highly selective Blood-Brain Barrier rather than a lack of efficacy
A protective barrier
Blood vessels in the brain are surrounded by the Blood-Brain Barrier (BBB), which consists of endothelial cells connected via tight junctions forming an effective physical barrier, along with pericytes and astrocytes supporting the maintenance of the brain microvasculature. This labyrinthine structure protects and maintains a stable environment in the brain and controls substances that can enter or leave the nervous system, including drugs.
More than 98% of all molecules with a molecular weight above 400 Da do not get through the barrier [1], except selective molecules, namely glucose, that are transported via membrane transporters using carrier mediated or receptor mediated pathways. Due to its restrictive nature, the BBB is the main obstacle preventing drugs, such as chemotherapies, immunotherapies, antibodies, and gene therapies from reaching therapeutic targets, limiting the efficacy and success of various Central Nervous System drugs.
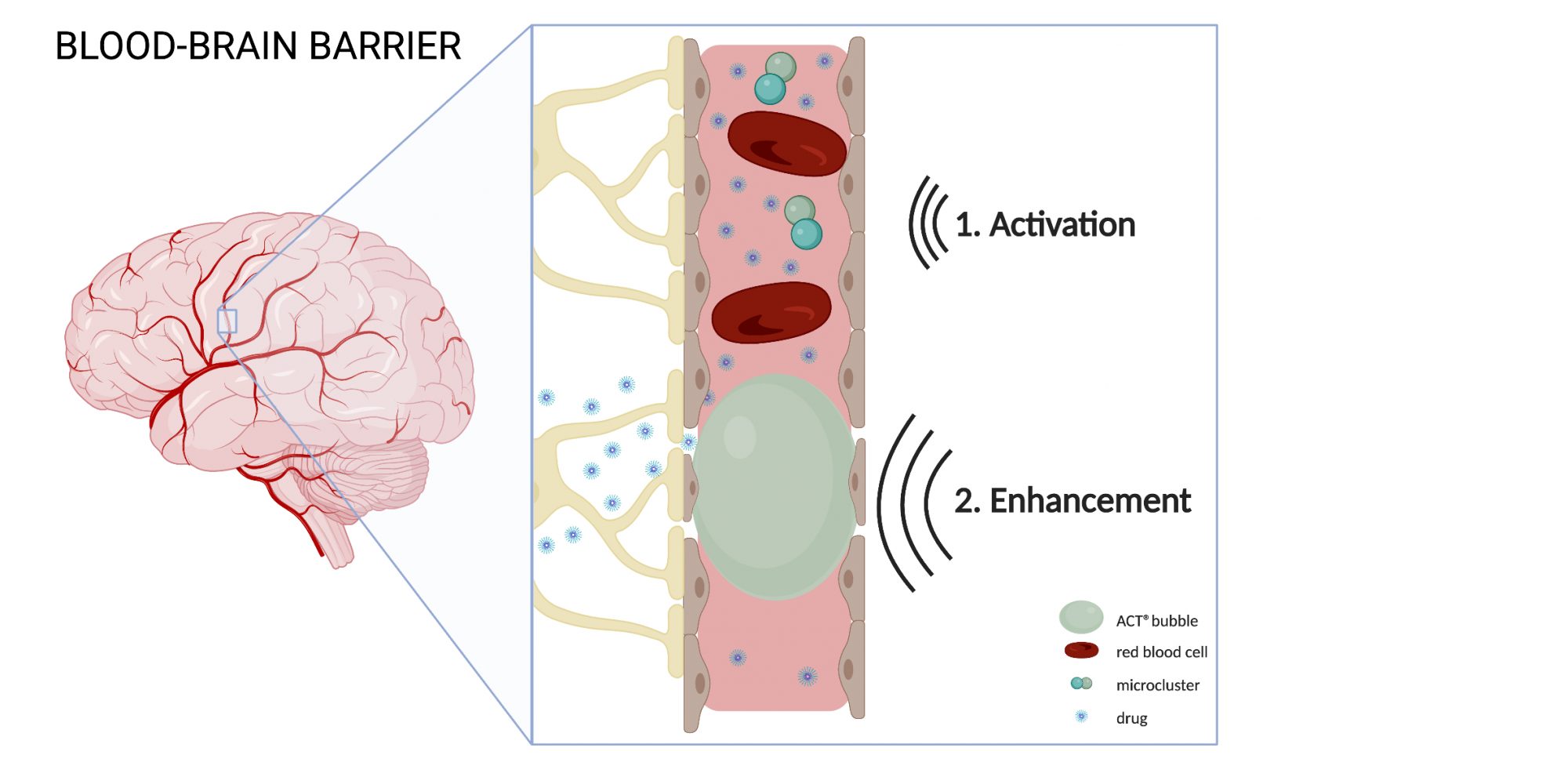
Enhancing drug transport across the BBB
Different methods such as chemical, biological, and physical stimuli have been tested to disrupt the BBB and enhance drug penetration transiently.
Researchers around the globe have shown preclinically and in clinical studies that the permeability of the BBB can be non-invasively increased by applying low-intensity ultrasound in combination with intravenous administration of microbubbles [2]. This emerging ultrasound-mediated BBB opening technique is greatly appealing as it is easily accessible, safe, and highly localized.
Most commonly used microbubbles are regular ultrasound contrast agents primarily designed and optimized for diagnostic purposes. These diagnostic microbubbles are small in size (1–3 μm in diameter) and free flowing in the vasculature, having only limited contact with the vessel wall and making them less efficient for therapeutic use.
New microbubbles for therapies
EXACT Therapeutics AS is providing a unique solution for targeted therapeutic enhancement. In an article in Open Access Government from July 2022 [3], we introduced our novel ultrasound and microbubble platform called Acoustic Cluster Therapy (ACT®), currently in Phase I Clinical Trial to investigate safety and tolerability (NCT04021277). Our ACT® bubbles address the shortcomings of current commercial microbubbles.
ACT® is composed of microdroplet-microbubble clusters, which achieve therapeutic benefit through a two-step insonation process called Activation and Enhancement. During Activation, ultrasound with a frequency of 2.7MHz is applied, inducing vaporization of the microdroplet, and forming large ACT® bubbles. Due to their large size (20-30μm in diameter), ACT® bubbles lodge temporarily in the capillaries, creating more contact with the endothelial cells. The following Enhancement step contains an application of low-frequency ultrasound (0.5MHz) that stimulates the ACT® bubble to oscillate in a controlled manner. The vibration induces mechanical forces on the capillary wall, increasing the local permeability of the vasculature, which in turn, improves the transport of the co-administered drug to the brain.
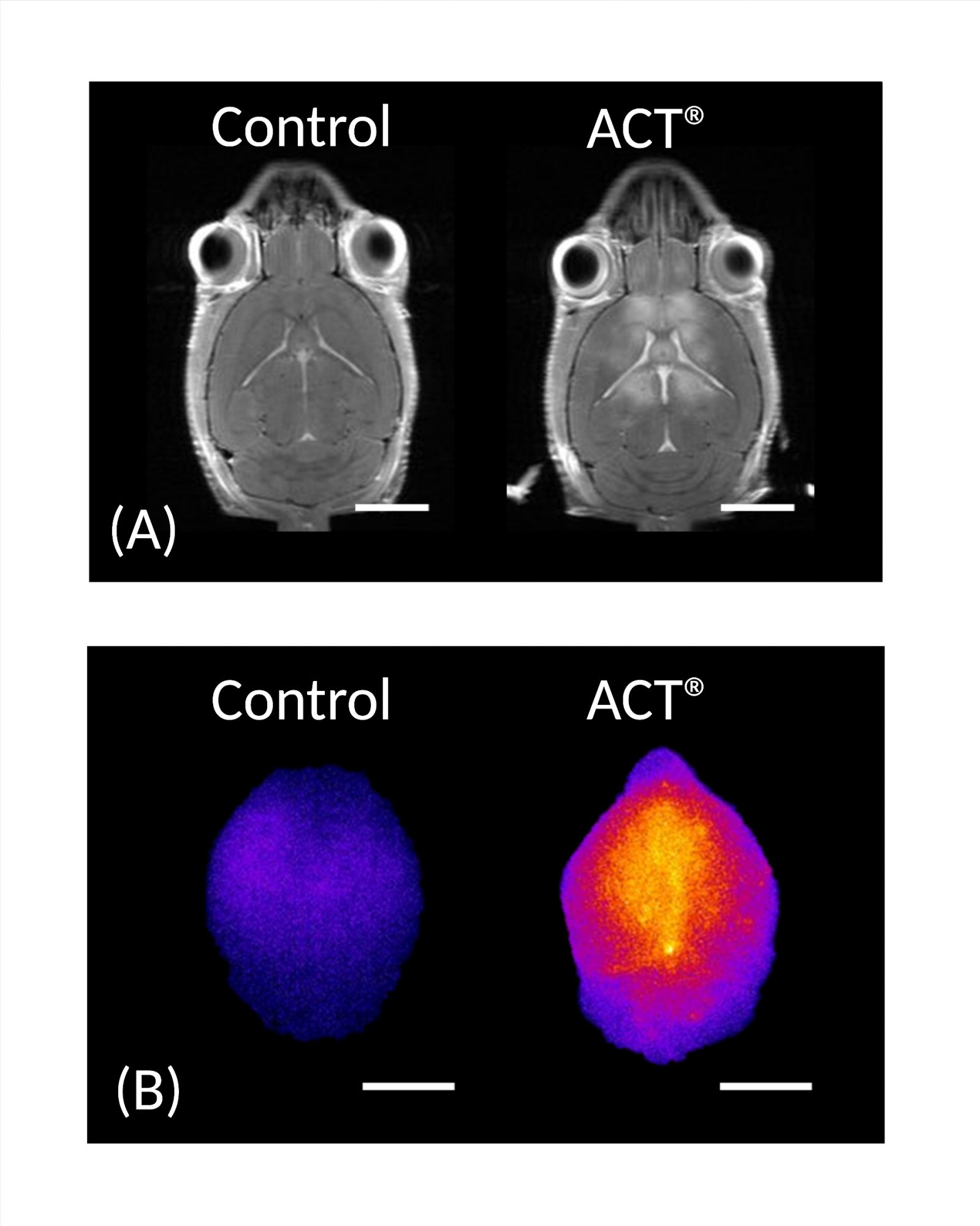
Supported by data
Preclinical studies of ACT® bubbles in the brain showed that ACT® could safely and transiently increase the permeability of this highly selective semipermeable barrier between the bloodstream and the extracellular space of the brain, enhancing the accumulation of co-injected model drugs with sizes up to 65 nm at the target site in the murine brain [4,5].
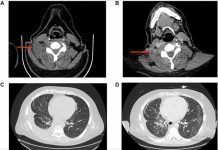
The increase in BBB permeability induced by ACT® was evaluated using contrast-enhanced magnetic resonance imaging (MRI), and increased accumulation was studied using near-infrared fluorescence (NIRF) imaging. MRI images showed a significant difference in the MR image intensity between control and ACT® treated brains, while NIRF showed a 5.2-fold increase in the accumulation of IRDye® 800CW-PEG, and a 3.7-fold increase of core-crosslinked polymeric micelles (CCPM) in the brain (Figure 2). Confocal laser scanning microscopy (CLSM) verified improved extravasation and penetration of CCPM into the brain parenchyma after ACT® treatment.
There were no apparent adverse events and histological examination did not show evidence of damage to the surrounding tissue. The BBB was fully restored 48 hours post-ACT® treatment, demonstrating ACT® as a gentle method to transiently increase the permeability of the BBB in a targeted area of the brain.
Future potential
ACT® holds great potential for treating various brain diseases, from brain tumours to Alzheimer’s disease, bringing medicines to their target site, and potentially reducing drug dosages. The immediate next step to demonstrate clinical benefit is testing in a relevant disease model.
References
- W.M. Partridge, Drug transport across the blood-brain barrier, J Cereb. Blood Flow Metab. 32 (11) (2012) 1959-1972.
- https://www.nationalgeographic.com/magazine/article/new-method-delivers-life-saving-drugs-to-the-brain–using-sound-waves
- https://edition.pagesuite-professional.co.uk/html5/reader/production/default.aspx?pubname=&edid=85f0d134-d2ec-4b73-b1ac-c5001c395a2a&pnum=20
- Åslund, A.K., et al., Efficient enhancement of blood-brain barrier permeability using Acoustic Cluster Therapy (ACT). Theranostics, 2017. 7(1) p.23.
- Olsman, M., et al., Acoustic Cluster Therapy (ACT®) enhances accumulation of polymeric micelles in the murine brain. Journal of Controlled Release, 2021. 337: p. 285-295.
Please note: This is a commercial profile.
© 2019. This work is licensed under CC-BY-NC-ND.