Phillip P. Chan, MD, PhD, Chief Executive Officer of CytoSorbents Corporation (NASDAQ: CTSO), discusses the use of CytoSorb, a broad-spectrum blood purification technology to treat cytokine storm and hyperinflammation in critically ill COVID-19 patients
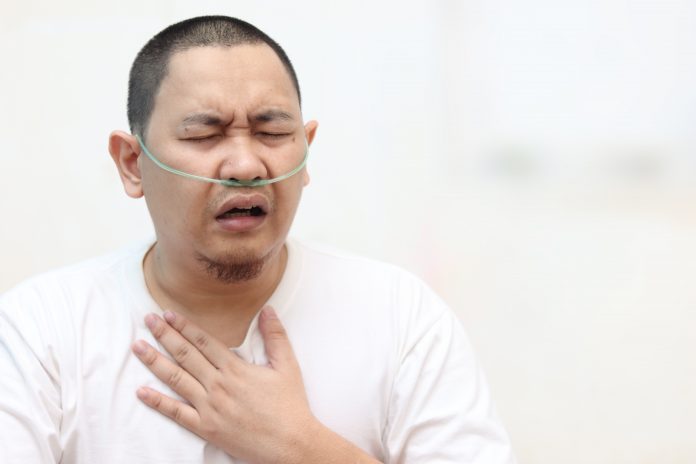
New COVID-19 infections continue to surge around the world, with the U.S., Europe, India, Russia, and Latin America bearing the most daily cases. The severity of illness in the current surge was initially low, but as numbers mount, so too have hospitalisations, intensive care unit (ICU) admissions and deaths. Severe respiratory failure requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO) has been the major cause of death throughout the pandemic, though shock, kidney failure and sudden cardiac death are also common complications. Intravenous dexamethasone, as a treatment of virus-induced inflammation, is the only therapy shown to improve mortality in mechanically ventilated patients, from 41% to 29%, in the U.K. RECOVERY study. However, that one in every three to every four people still die despite dexamethasone highlights the complexity of COVID-19 infection and the need for additional therapies.
Severe inflammation and cytokine storm can kill
Inflammation is the body’s normal adaptive response to injury and infection. However, every year, millions of people die from complications of severe, uncontrolled inflammation triggered by common life-threatening conditions such as infection and sepsis, influenza, trauma, surgical complications, liver failure, burn injury, and many others. This inflammation is often driven by a “cytokine storm,” or the excessive production of cytokines – a class of more than 100 different immune system messengers that normally regulate and activate the immune response. Left unchecked, this cytokine storm and the inflammation that ensues, can rapidly kill cells and damage vital organs like the lungs, heart and kidneys, often leading to organ failure and death. In fact, organ failure is responsible for nearly half of all deaths in the intensive care unit (ICU), and has made sepsis (the overzealous immune response to an infection) the #1 killer worldwide, contributing to one in every five deaths each year.
A “tale of two types of lung injury” due to hyperinflammation in severe COVID-19 infection
In COVID-19 infection, aggressive hyperinflammation has been directly correlated with greater lung injury and other complications, the need for mechanical ventilation and the risk of death. It is believed that hyperinflammation and damage to the blood vessels in the lungs play an important role in this “tale of two types of lung injury.” On one hand, cytokine storm and other inflammatory toxins can cause a breakdown in the integrity of blood vessels in the lung. This allows fluid and cells from the bloodstream to flood the delicate air sacs of the lung, essentially drowning the patient from the inside out. At autopsy, this fluid has led to wet, heavy lungs that are four times the weight of normal lungs.
On the other hand, damage to the blood vessel wall from viral infection, cytokine storm, and likely other inflammatory toxins is thought to lead to a clotting cascade, where the delicate balance between bleeding and clotting is tipped in favour of clotting. In this hypercoagulable state, blood clots can form in large veins that often travel to the lungs, resulting in pulmonary emboli, while blood clots also form in tiny blood vessels of the lungs and other organs, leading to a compromise of blood and oxygen delivery, resulting in widespread organ damage. In the lungs, these clots can prevent blood from reaching the air sacs of the lung, resulting in low oxygen levels in the blood. Many critically ill patients exhibit both pathologies, resulting in an unusually severe acute respiratory distress syndrome (ARDS) and hypoxia that many had not seen before.
CytoSorb® reduces the “fuel to the fire” of inflammation
CytoSorb is a powerful broad-spectrum blood purification cartridge approved in the European Union, manufactured in the U.S., and distributed in 66 countries worldwide, to reduce “cytokine storm” and other inflammatory toxins or “evil humors” that can otherwise cause uncontrolled massive inflammation, organ failure and death in common critical illnesses. These are conditions where the risk of death is extremely high, yet few if any effective treatments exist. CytoSorb® has now been used in more than 110,000 human treatments to date across critical care and cardiac surgery, establishing itself as a leading therapy to safely treat cytokine storm and its complications.
How does CytoSorb® work?
Within each CytoSorb cartridge are hundreds of thousands of tiny porous polymer beads, roughly the size of a grain of salt. Each of these beads has millions of pores and channels that allow the bead to act like a tiny sponge and remove cytokines and other inflammatory mediators of a certain size. Treatment is very simple: a temporary dialysis catheter is placed into a major vein, blood is pumped out of the body by a standard dialysis or ECMO machine found in ICUs around the world, through the CytoSorb cartridge where cytokines and other toxins are removed, and the purified blood is then recirculated back into the patient over and over again. A single cartridge can treat a patient’s entire blood volume more than 70 times in a day, with a new cartridge used each day. Most patients require one to five cartridges, depending on the illness.
Use of CytoSorb in critically ill COVID-19 patients
Although CytoSorb is used in many different life-threatening conditions, one of the most pressing needs is to help critically ill patients with organ failure complications in severe COVID-19 infection. The rationale of using CytoSorb in these patients is to help remove the circulating inflammatory toxins generated by SARS-CoV-2 viral infection that are continuously damaging the lung, ultimately giving the lungs a chance to heal. We believe this healing is the key to helping patients wean from mechanical ventilation and extracorporeal membrane oxygenation (ECMO) faster.
CytoSorb has already been used to help treat more than 3,000 critically-ill COVID-19 patients across more than 30 countries. When used early and aggressively, CytoSorb has demonstrated a consistent ability to reduce inflammatory mediators, reverse shock, and to help improve lung function and weaning of COVID-19 patients from mechanical lung support in case series that have been published or presented at conferences.
In April 2020, CytoSorb received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for use in adult, critically-ill COVID-19 patients with imminent or confirmed respiratory failure, with many U.S. hospitals now using the therapy. We are currently collecting U.S. treatment data with our CTC COVID-19 registry. CytoSorb has also been included in national COVID-19 treatment guidelines in many countries around the world.
Other applications
In addition to cytokine adsorption, CytoSorb has received CE-Mark label expansions for the removal of bilirubin (liver disease), myoglobin (trauma), and the antithrombotic drugs, ticagrelor (Brilinta® or Brilique®) and rivaroxaban (Xarelto®), during urgent and emergent cardiothoracic surgery. CytoSorb has also been granted U.S. FDA Breakthrough Designation for the removal of ticagrelor during emergent and urgent cardiothoracic surgery.
CytoSorbents’ technologies have received non-dilutive grant, contract, and other funding of more than $38 million from the U.S. National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI), the Defense Advanced Research Projects Agency (DARPA), the U.S. Department of Health and Human Services, the U.S. Army, Air Force, Special Operations Command (SOCOM), Air Force Material Command (USAF/AFMC), and others. The Company has numerous products under development based upon this unique blood purification technology protected by many issued U.S. and international patents and multiple applications pending, including ECOS-300CY™, CytoSorb-XL™, HemoDefend-RBC™, HemoDefend-BGA™, VetResQ™, K+ontrol™,
ContrastSorb, DrugSorb, and others. For more information, please visit the Company’s websites at http://www.cytosorbents.com and www.cytosorb.com, or follow us on Facebook and Twitter.
*Please note: This is a commercial profile