Nigel Shrive and David Hart from the McCaig Institute for Bone and Joint Health, University of Calgary, unravel the complexity of osteoarthritis and stress the need to integrate innovation in biomechanics, biology and imaging
The more we learn about osteoarthritis (OA) the more we realise that it is not a single disease, but a complex set of diseases from multiple aetiologies but with a common endpoint – loss of cartilage from the surface of bones at an articulating joint. This common endpoint develops in humans who are very heterogeneous, who have, for example, different genetics, post-natal development, nutrition, activity levels and weight.
Understanding osteoarthritis
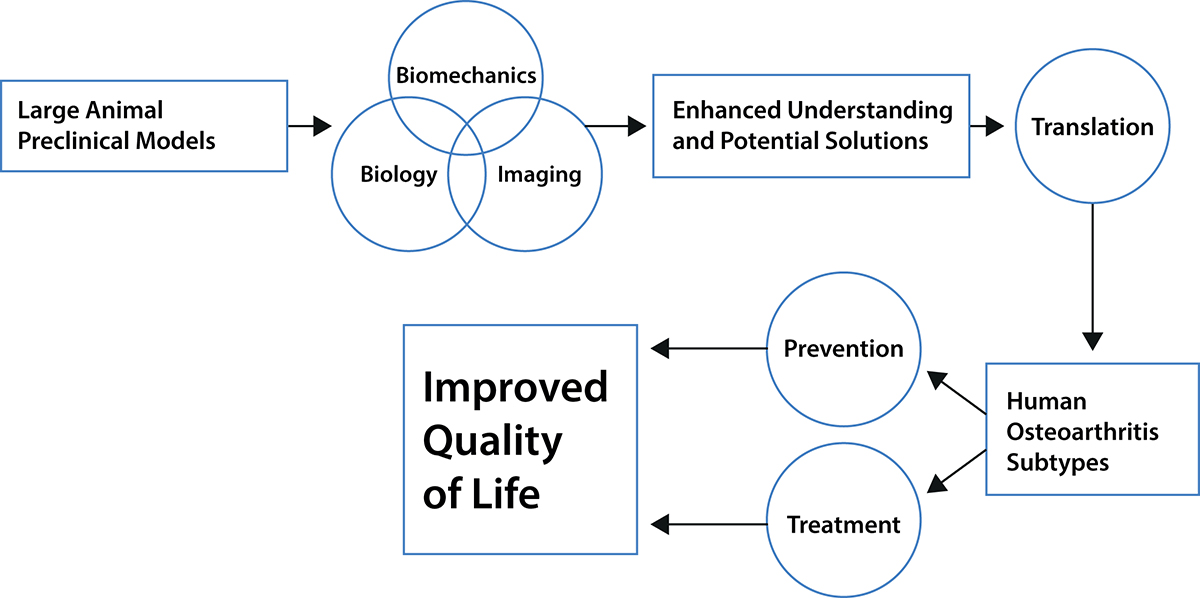
In the past, investigators approached understanding OA based on their individual expertise which was usually either in some aspect of biomechanics or biology. This has unfortunately led to many important questions remaining unanswered due to too narrow a focus of investigation or limitations of the methods employed. To combat this, we developed a methodology for measuring the changes in kinematics very accurately in a sheep model, together with the capability of reproducing those motions, again very accurately, with a robotic test system from which we could determine tissue loads.
More recently, we added the ability to measure stress in the joints using Fibre Bragg Grating (FBG) sensors in a fibre optic cable the approximate diameter of a human hair to obtain very sensitive biomechanical information. We can also measure various biological factors in this model and thus integrate the biomechanical and biological findings. Our objective is to document the sequence of biomechanical and biological changes over time after a surgically induced injury to the joint to obtain an understanding of the causes of post-traumatic OA initiation and progression. The sheep that are used are heterogeneous similar to humans, and like humans respond individually to the injury. There is far more we need to comprehend – for example, the role of genetics and epigenetics – why does the disease progress more rapidly in some animals compared to others?
We have induced various injuries surgically to the ovine stifle joint and studied the changes in motion, loads and some biological factors over time. The biological factors are histology (the structural integrity of cartilage in particular), gene expression (what molecules are the cells being stimulated to produce) and protein (what molecules are actually produced). Joint kinematics are determined before and at 10 and 20 weeks after surgically induced injuries aimed at giving differing levels of instability in the joint. Motions measured during gait on a treadmill are reproduced by our robotic test system with an accuracy of 0.1 mm and 0.1 degrees. Loads in individual structures between the bones are determined by removing those structures one-by-one and repeating the joint motions with high fidelity, invoking the principle of superposition. Stress measurements are determined from the FBG sensors in fibre optic cables. These cables are so small that we do not believe they alter the lubrication or load transfer mechanisms in the joint during the in vitro testing. We use each animal as its own control in an N of 1 experimental design.
Different components of the initial acute inflammation from the surgery/ injury diminish after a few days to weeks, but there are at least two important consequences. One is that cytokines that are known to contribute to the degradation of cartilage are upregulated, and the second is that the constituency of the synovial fluid that supports the health of cartilage is significantly disrupted. The high molecular weight Hyaluronic Acid (an accepted important molecule in the lubricating effect of synovial fluid) is initially depleted, and the dynamic coefficient of friction is doubled. In our sheep model, it takes some 10 weeks before the synovial fluid is restored to its original constituency and the coefficient of friction returns to its original value.
Meanwhile, there have been changes to the kinematics of the joint – the way the bones move relative to one another during gait, as well as to the loads in the various tissues between those bones (the menisci, the cartilage and ligaments). While changes are on-going at 20 weeks, we have found no correlation between kinematic measures in the usual 6 degrees of freedom defined clinically for a joint and the development of osteoarthritic-like changes in the joints. If the kinematics are expressed in terms of helical axis variables, the correlations are somewhat better but remain weak. However, when kinetic changes are examined – specifically increases in stress on the cartilage surface, there appears to be consistency with the development of focal damage being close to sites of the largest increase in either average or peak stress.
Our results must be interpreted with care as the biomechanical data are obtained at limited time points for walking gait only, without knowledge of the loading that may occur from other activities and in the intervening time periods. Nevertheless, the data do point to where further studies would provide insightful information as to the potential causes of post-traumatic and possibly idiopathic OA. The stresses and relative surface motions at various locations in the joint are changed substantially by the injury. We see that the stress perpendicular to the cartilage surface is increased substantially at some locations. Given the surfaces move one relative the other, albeit, through very small movements during the stance phase of the gait cycle, the associated friction will also be increased and potentially altered in direction. The frictional stresses are increased beyond just the change in perpendicular stress over the first few weeks after injury because the coefficient of friction is also increased.
When we think of the effect of this change on the cartilage, we recognise that the orientation of the collagen fibres in the Superficial Tangential Zone (the zone immediately below the Lamina Splendens – a very thin layer of interwoven collagen fibres at the surface of the tissue) is laid down as joints develop and the bones move relative to each other. The fibre orientation is probably arranged to resist the most frequent higher loads from that individual’s movements. If the friction is now greater and at a large angle to the fibre orientation, one can easily imagine that the new stress regime will pull the fibres apart and the surface will exhibit a fibrillated appearance. A simple analogy is a piece of wood – it is very strong if you pull in the direction of the grain, but rips apart relatively easily if you pull perpendicular to the grain.
Another factor we acknowledge is that materials science teaches us that no material is perfect in its structure. All materials contain flaws. There is absolutely no reason to suppose that cartilage is any different. The cells produce the molecules that constitute the material of the structure – collagen, glycosaminoglycans and others – and these molecules self-assemble into the structural form of the tissue. Perfection cannot be achieved everywhere. Thus, while we do not know what would constitute a flaw in the complex structure of cartilage, we recognise that flaws in regions of a high step change in stress will now be the potential source of local failure. In addition, the size of any flaw could be exacerbated by the cytokines upregulated by the acute inflammation because they will be sites of higher than normal local energy: or indeed, the cytokines could facilitate the creation of flaws.
The two established possibilities of increasing damage from a flaw are from a peak stress resulting in crack extension or from repeated loading inducing incremental crack growth in each cycle (fatigue). The first possibility is related to the change in peak stress – has the new peak stress during activity reached a level which would cause a crack to extend from the flaw? The second possibility of incremental crack growth could also arise from the peak stress being repeated, but is significantly affected by the mean stress – we used the average stress per cycle during gait as a surrogate. In our studies, focal damage of the cartilage was consistently found close to a sensor with the biggest change in one or other of these two measures.
Osteoarthritis research
In osteoarthritis research, it is accepted that metabolism in cartilage is slow and the tissue is unable to adapt quickly to new mechanical environments. It does not matter what the aetiology of the arthritis is, the cartilage can’t adjust its structure fast enough and the new stresses slowly disrupt the structure. This situation results in the development of chronic inflammation, particularly at the level of the synovium which further aggravates the damage. There is clearly an intricate interplay between the mechanical and the biological changes that occur in the joint over time from a known injury.
Integrating and understanding the consequences of the sequential biological and biomechanical changes in a joint, therefore, appears necessary to understanding the development and progression certainly of post-traumatic OA. Such integration will only be achieved from comprehensive studies where both biological and biomechanical changes in a joint are followed over time. We realise the studies we have undertaken are not sufficiently comprehensive – we need to add at least genetics, epigenetics and muscular activities. Muscle activity needs to be considered because muscles are central to movement and mobility: however, muscle activity changes with age (sarcopenia or injury, and menopause in females). Imaging needs to be employed to measure changes in bone shape and validated models need to be developed to explore how the changing shapes and motions affect the stresses in the cartilage.
As bone is a reactive tissue and changes in response to alterations in loading, such imaging results would provide additional insights into the dynamics of the joint as an organ system and as such, could provide insights into early changes occurring before the joint becomes symptomatic with pain (as an indicator of loss of functional integrity). Genetic and epigenetic factors need to be recorded such that machine learning and artificial intelligence techniques can be used hopefully to reveal the combinations of factors that predispose a joint to become arthritic or at risk to do so.
All of these measures need to be made at a level of accuracy for meaningful analysis (such as the kinematics and stress measures here). This can only be done with a large animal model. What has happened to date is that experts have looked at the equivalent of a carburettor, or the flame motion after the spark plug has fired, or how a shock absorber works – on their own, none will reveal how the car is put together and works. We believe that it is only through a comprehensive approach as we describe that the causes of post-traumatic OA and potentially idiopathic OA can be revealed.
The development of idiopathic knee OA
As the majority of OA patients develop their OA due to reasons or causes that are presently unknown (i.e. idiopathic), there is a real need to look beyond the study of knee injury as the instigator of OA development. Based on the well characterised instrumented sheep knee described above, we believe this platform is now well-positioned to be used to address many of the challenging questions surrounding the development of idiopathic knee OA. These include, but are not limited to:
- What is the role of specific muscle weakness in OA development?
- How do growth and maturation variables contribute to OA risk?
- Does ageing affect the biomechanics, biology and imaging characteristics of the knee?
- As females are more likely to develop OA after menopause, how does the loss of hormones contribute to risk for OA development?
- How does obesity contribute to knee OA development?
- Why do some sheep, and some people, never get OA even when elderly?
The answers to such questions require a complex, but integrated research effort with preclinical models that are relevant to human disease. The instrumented sheep knee and sheep, in general, fit the characteristics necessary to gain information that can be translated to the human research effort to prevent or effectively intervene in the development of the ever-expanding population at risk for this disease.
Please note: This is a commercial profile
© 2019. This work is licensed under a CC BY 4.0 license.