Energy defects, neuroinflammatory processes, and abnormal cellular morphology in neurodegenerative diseases (ND) would constitute extremely informative brain imaging biomarkers of disease progression and readouts in clinical trials. Emerging research aims at developing novel brain imaging methods to study these different aspects not only in animal models but also in patients through a translational process of research. These innovative imaging tools should help to discover new functional biomarkers to be used in clinical trials.
Neurodegenerative diseases: challenges
Neurodegenerative disorders represent a major social and economic burden in Europe. Because the incidence of these devastating illnesses is dramatically increasing with age and the European population is ageing, this current health problem is going to dramatically evolve toward an unsustainable situation in the next decades. This is one of the major challenges of EU research programs for innovation in medicine. Understanding the etiology of these diseases which likely stems from complex interactions between environmental (pathogens, food, exercise, stress etc…) and genetic risk factors, is crucial to develop novel therapies aimed at curing patients, prevent these disorders, or at least retard the onset of symptoms. The validation of predictive biomarkers of disease progression and severity, especially at the brain level is crucial to improve the design of clinical trials
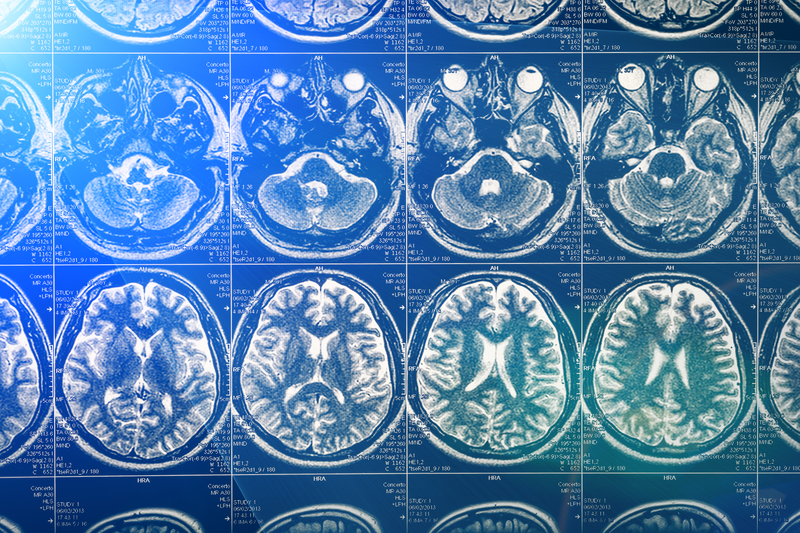
Why brain imaging matters Brain imaging methods already exist to study brain dysfunction and degeneration in ND, such as for example Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and multiple sclerosis. However, these methods need to be further developed to provide information that is more relevant to the complex biological processes underlying the pathogenesis of these illnesses. For example, some biological processes are encountered in almost all ND such as abnormal aggregation of protein (e.g. senile plaques and neurofibrillary tangles in AD-related dementia), loss of region-specific neuronal markers, neuroinflammation and early energy defects. In addition, specific imaging tools and methods are yet to be developed to better determine the biological efficacy of novel therapeutic interventions. To this purpose, it is necessary to develop “probes” that can inform the clinician whether the treatment is actually biologically active. This is a prerequisite before launching clinical trials with large patient cohorts to determine the efficacy of the therapy in terms of symptoms and a patient’s quality of life amelioration.
Examples of novel imaging tools
Several imaging methods can be developed to better explore multiple biological processes in the brain of patients. The term “methods” encompass the device (e.g. Scanner) and an object of biological interest that can be detected. The object is either naturally found in the brain (e.g. glucose, or ATP) or is a molecular “probe” that specifically concentrates in a brain region and indicates a specific biological process. When a process is found to be changed in patients at a given stage of the disease as compared to healthy volunteers, this can be called a biomarker. The imaging biomarker is especially interesting when it correlates with some of the neurological or psychiatric symptoms. The development of new device/probe couples makes possible the discovery of novel, more relevant biomarkers.
Many methods can be first developed in animal models. For technical or safety reasons, these methods cannot always be translated to the clinic. However preclinical in vivo approaches can accelerate the research process because they increase the power of analysis in terms of spatial and /or time resolution. For example, pioneering studies showed that two-photon microscopy can be used to analyse the abnormalities of neuronal dendritic spines (that are cellular structures smaller than one micron) in mouse models of Alzheimer’s disease. Other non-invasive preclinical imaging methods utilise detection devices that are comparable to those used in the clinic such as Positron Emission Tomography (PET), Magnetic Resonance Imaging (MRI) or Nuclear Magnetic Resonance spectroscopy (NMRs). After adaptation and optimisation, many of these imaging methods (device/probe) can be translated to the clinic to explore very specific biological processes.
These methods permit the follow up of brain degeneration in a cohort of animals. This represents a real ethical advantage to reduce the animal numbers in experiments. Some of the most promising imaging approaches currently developed in our laboratory can be briefly presented.
Looking at the microscopic scale using novel diffusion NMR spectroscopy is possible thanks to high magnetic fields combined with other improvements of MRI devices. It gives access to the study of groups of brain cells and to subtle morphological abnormalities. DWI (diffusion water imaging) gives access to the movement of water molecules along axons and myelinated fibres and thus, the organisation of white matter and the connectivity within the different region of the brain. Another promising method, called CEST imaging (Chemical Exchange Saturation Transfer) characterises the specific biophysical properties of the water protons that change depending on the biological molecules (e.g. glucose or glutamate) or chemical “probes” (CEST agents) to which they bind. CEST imaging gives brain maps of these molecules in the brain. NMR spectroscopy can be used to detect different nuclei (e.g. 1H, 31P, 13C and 17O) and this allows studying the biochemistry of the brain (e.g. concentrations of metabolites). NMR spectroscopy permits to measure complex enzymatic reactions, especially those related to energy metabolism (e.g. the rate of synthesis of ATP in the brain). Very innovative approaches that the measurement of diffusion of brain metabolites by 1H NMR could permit to quantitatively determine the pathological changes in the microscopic architecture of neurons and astrocytes in ND.
In addition to NMR, nuclear medicine approaches can also greatly contribute to study very specific molecular processes. Novel radiotracers for PET that target biological processes involved in the pathogenesis of ND are being developed (e.g. specific radiolabelled probes revealing abnormal protein aggregation in patients). Novel radiopharmaceutical probes can be developed to study membrane receptors or different types of enzymes genetically involved in ND (e.g. Presenilin, LRRK2) and that are targets of innovative drugs.
Other types of cutting edge developments are emerging in technologies for image processing. The lab also focusses on technological breakthroughs allowing tridimensional (3D) co-registration and analysis of multiple modalities of imaging (at different scales). This has a high potential to characterise the efficacy of a treatment in animal models.
In summary, the innovation in imaging should significantly accelerate the discovery of biomarkers to improve the diagnosis and follow up of patients and optimise the evaluation of novel therapies in clinical trials.
Emmanuel Brouillet
Molecular Imaging Research Centre (MIRCen)
www-dsv.cea.fr/dsv/instituts/institutd-imagerie-biomedicale-i2bm/services/mircen-mircen