Prof Dr Regina Fluhrer from the LMU & DZNE in Munich and Prof Dr Bernd Schröder from the TU in Dresden, Germany, explain how intramembrane proteases contribute to the proper development of immune cells
The immune system is crucial to protect organisms from a variety of pathogens, including bacteria, viruses and fungi. However, the development and function of immune cells need to be tightly controlled in order to avoid reactivity against the host, which is termed autoimmunity. A variety of common diseases, including rheumatoid arthritis, multiple sclerosis (MS) and inflammatory bowel disease (IBD) are caused by an overshooting activity of the immune system and inflammatory reactions. Many drugs commonly used to suppress the immune system in those patients like corticosteroids which act in a very general way and have significant side effects when long-term treatment is required. Despite major advances over the last few years, there is still a demand for therapeutic strategies targeting specific components of the immune system.

In this context, it was an important finding that certain intramembrane proteases, which are druggable enzymes and can be targeted by small molecule inhibitors, control the development and function of certain immune cells. Intramembrane protease represent enzymes embedded in cellular membranes which can cleave the transmembrane domain of membrane-spanning substrate proteins (for more details see the October 2018 edition of Open Access Government). One of these intramembrane proteases is Signal peptide peptidase-like 2a (SPPL2a) which is localised in the membrane of lysosomes, an intracellular compartment involved in degradation of proteins and other macromolecules. With its ability to cleave transmembrane proteins, SPPL2a contributes essentially to the turnover of these proteins.

Although the discovery and characterisation of further SPPL2a substrates is still ongoing, one substrate of particular importance in immune cells is the CD74 protein which has several well-established functions in B lymphocytes and dendritic cells (DCs). Whereas the latter are particularly important to recognise invading pathogens, B cells produce antibodies targeting the “intruders” which is a major mechanism to clear infections.
A specific step in the degradation of the CD74 protein requires intramembrane proteolysis by SPPL2a. Therefore, in absence of the protease a small, membrane-embedded fragment of CD74 massively accumulates in B cells and DCs and disturbs their development and function. SPPL2a deficiency in mice results in a major impairment of both cell types and in humans, it causes increased susceptibility to infections with mycobacteria like tuberculosis (TB). The reduced ability of humans with mutations in the SPPL2a gene to fight mycobacterial infections can be explained by a loss of specific DCs, which are required to trigger immune responses against these pathogens.
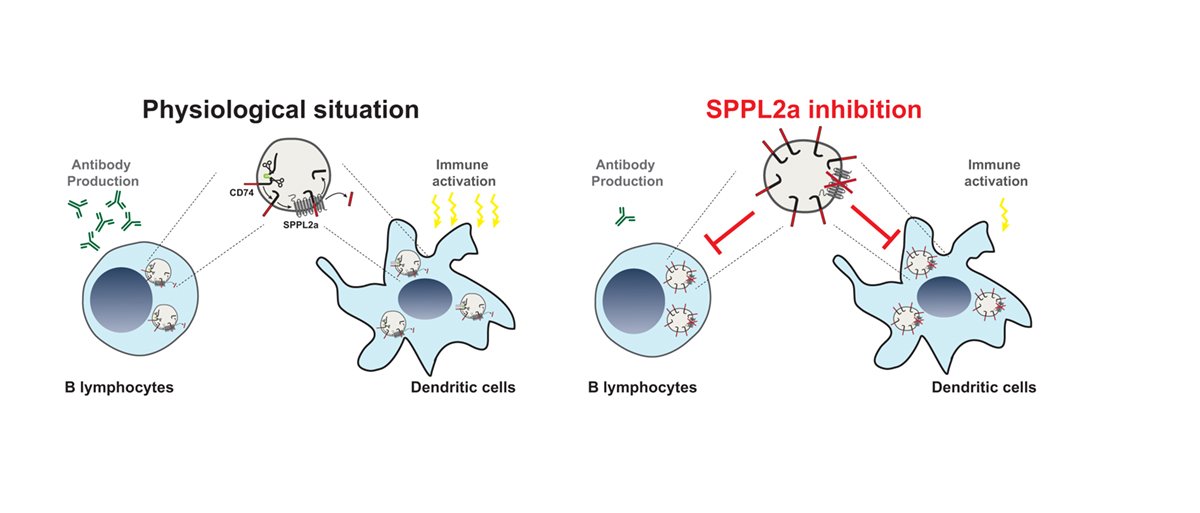
Thus, the presence of SPPL2a and its ability to degrade CD74 represents an important checkpoint in B cells and DCs. The dependence of these cells on SPPL2a may allow to deplete them in patients with SPPL2a specific inhibitors, which could be beneficial in different disease contexts like MS or Systemic lupus erythematosus (SLE) and represents an interesting novel concept.
Besides ensuring the proper maturation of immune cells, it is also important to tightly control their number, since the unrestricted proliferation of, for instance, B cells results in diseases like multiple myeloma. One protein that is involved in the regulation of B cell survival is the B-cell maturation antigen (BCMA). It is expressed on the surface of B cells and is cleaved by the γ-secretase complex that contains the intramembrane protease presenilin as an active subunit (for more details see July 2019 edition of Open Access Government). The inhibition of this cleavage results in accumulation of BCMA and an increased number of B cells in the bone marrow, indicating that an intramembrane protease is critically involved in an immunoregulatory mechanism to limit B cells in bone marrow and most likely also in plasma.
SPPL3, another intramembrane protease and homologue of SPPL2a, is crucial for normal development of Natural Killer cells (NK cells). NK cells are part of the innate immune system and critical for tumour surveillance as well as clearance of virally infected cells. After development in the bone marrow, NK cells migrate and populate all organs of the body where they undergo final maturation steps and become fully active “guardians”. Deficiency of SPPL3 in mice results in impaired maturation of NK cells and reduced clearance of tumour cells. This makes SPPL3 an interesting player in the immune system and in tumour defence. However, before SPPL3 is considered as a therapeutic target, it needs to be further investigated if it is indeed dysregulated in immune deficiencies or malignant tumours.
As these examples show, several intramembrane proteases play pivotal roles in specific immune cells. It seems that their activity represents critical checkpoints which these cells need to bypass in order to differentiate and become functionally mature. Certainly, the mechanisms involved in the differentiation processes will require further work to understand them at the molecular level. But previous research has unambiguously shown that defining the action of intramembrane proteases in immune cells will increase our general understanding of how we fight infections and the way in which this may be exploited to control an over-activated immune system.
References
Mentrup T et al. Signal peptide peptidase and SPP-like proteases – Possible therapeutic targets? Biochim Biophys Acta. 2017 1864, 2169-2182.
Schneppenheim et al., The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J Exp Med 2013, 210, 41-58.
Kong et al., Disruption of an antimycobacterial circuit between dendritic and helper T cells in human SPPL2a deficiency. Nat Immunol 2018, 19, 973-985.
Hamblet et al., NK Cell Maturation and Cytotoxicity Are Controlled by the Intramembrane Aspartyl Protease SPPL3. J Immunol. 2016 Mar 15;196(6): 2614-26
Meinl et al., Shedding of BAFF/APRIL Receptors Controls B Cells. Trends Immunol. 2018 Sep; 39(9): 673-676.
Prof Dr Regina Fluhrer
Biomedical Center (BMC),
Ludwig Maximilians University of Munich (LMU) & German Center for
Neurodegenerative Diseases (DZNE)
Tel: +49 89 4400 46505
regina.fluhrer@med.uni-muenchen.de
www.biochemie.abi.med.uni-muenchen.de/fluhrer_lab/index.html
Please note: this is a commercial profile