Jakob Birkedal Wagner of the DTU Center For Electron Nanoscopy discusses research into low-dimensional nanostructures from carbon assembly
The abundance of carbon in nature combined with the variety of assembling the individual atoms into nanostructures with various physical properties such as metallic, semiconducting, semi metallic etc. make carbon nanostructures of particular interest and importance in tomorrow’s electronic and optical devices. Single walled carbon nanotubes and graphene show enormous potential in electronic devices and are already incorporated as transparent conductors in touchscreens to name but a few applications.
Single walled carbon nanotubes
Single walled carbon nanotubes (SWCNT) consist of a hexagonal lattice of carbon atoms rolled into a tube. The specific diameter and chirality (or helicity) of the carbon tube determine whether the nanotube acts metallic or semiconducting. Tubes are typically grown from metallic seed nanoparticles (Fe, Ni, Co, etc). In order to grow the nanotubes selectively (with a specific chirality) for efficient application, a better understanding of the actual growth mechanism is essential. Watching the carbon structure form at the atomic scale gives valuable insight in the key parameters and processes determining the diameter and chirality of the carbon nanotubes and thereby their electronic and optical properties. In situ growth of such carbon structures by means of environmental transmission electron microscopy (ETEM) is a unique opportunity to follow the growth live [1]. At Center for Electron Nanoscopy at the Technical University of Denmark, such growth studies have successfully been carried out in the past years.
From gaseous carbon to solid carbon
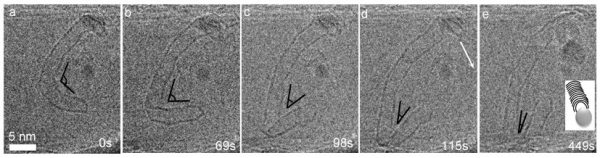
Figure 1 shows the transition from gaseous carbon to solid carbon forming CNTs. The elongation process of a SWCNT is shown by a series of transmission electron micrographs extracted from a movie, acquired during exposure of a Co/MgO sample to a mixture of CO and H2 at elevated temperature [2]. The diameter and thereby the chirality of the SWCNTs strongly depends on the access to carbon atoms, which can be incorporated into the tube securing growth. Changes in the amount of accessible carbon either by changes in the carbon supply (gas pressure of CO) or by changes in the rate of catalytic cracking of CO to free carbon atoms, strongly influence the growth and can be a limiting step for the CNT growth. External forces such as stress due to a high density of CNTs will also lead to growth termination, because in this case, the incorporation rate of active carbon atoms into the tube is limited. However, we observe that the same catalyst particle stayed active in terms of nucleating additional solid carbon structures after the growth termination of the first SWCNT. These observations elucidate the importance of an in-depth understanding of the role of catalysts and carbon sources in the continued growth of SWCNTs.
References:
1: M. He, H. Jiang, B. Liu, P. V. Fedotov, A. I. Chernov, E. D. Obraztsova, F. Cavalca, J. B. Wagner, T. W. Hansen, I. V. Anoshkin, E. A. Obraztsova, A. V. Belkin, E. Sairanen, A. G. Nasibulin, J. Lehtonen, and E. I. Kauppinen, Scientific Reports 3, 1460 (2013)
2: L. Zhang, M. He, T. W. Hansen, J. Kling, H. Jiang, E. I. Kauppinen, A. Loiseau, J. B. Wagner, ‘Direct Evidence for Growth Termination and Multiple Nucleation of Single-Wall Carbon Nanotubes’, ACS Nano, 11, 4483, (2017)
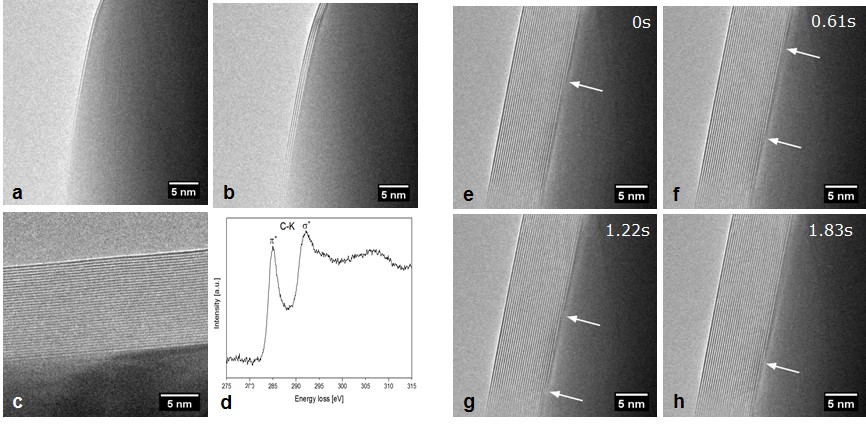
3: J. Kling, T. W. Hansen, J. B. Wagner, ‘Quantifying the growth of individual graphene layers by in situ environmental transmission electron microscopy’, Carbon 99, 261 (2016)
For more information, visit: www.cen.dtu.dk/english