The NHS have gained a new drug, atezolizumab, which can give 34% protection against lung cancer recurrence or death
The NHS treatment is targeted against non-small cell lung cancer (NSCLC).
According to data from Cancer Research UK, 80-85% of cancers in the UK are non small cell lung cancers. The three main types are adenocarcinoma, squamous cell carcinoma and large cell carcinoma. They’re grouped because it means they can react similarly to treatment.
Health and Social Care Secretary Sajid Javid said: “The UK is renowned for its ability to find and deploy the most innovative treatments the world has to offer to NHS patients. This breakthrough will be life-changing for hundreds of people and marks a significant development in our war on cancer.”
What is Atezolizumab?
Atezolizumab is the first immunotherapy approved for patients with early-stage NSCLC whose tumours express the PD-L1 mutation, and who have undergone surgery and chemotherapy.
Essentially, the drug can dramatically lessen the risk that tumours will come back after patients go through intensive treatment – while improving the likelihood that this disease will be cured in the long-term.
Clinical trials have shown atezolizumab, known commercially as Tecentriq, can reduce the risk of cancer recurrence or death by 34% – following surgery and chemotherapy.
How does it work?
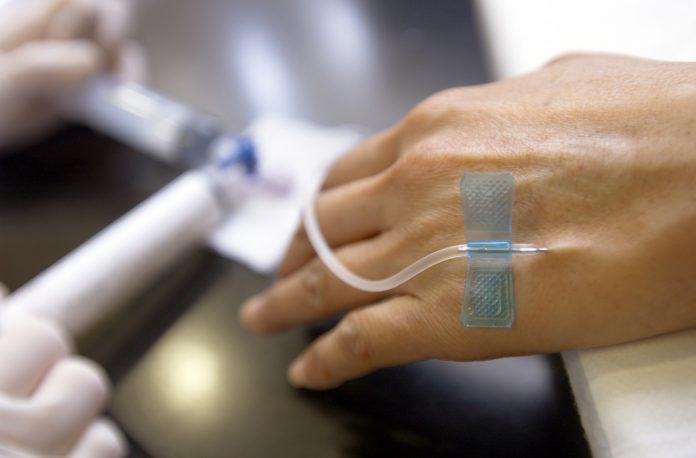
Given as an IV drip, this innovative treatment works by blocking a protein that stops the immune system from attacking cancer cells, by making cancer cells more visible to the immune system.
Professor Charles Swanton, Cancer Research UK’s chief clinician, said: “It’s great to see this drug approved so quickly, bringing even more optimism to tackling a difficult to treat cancer.
“Sadly, early-stage lung cancers often come back after surgery, especially larger tumours, and the approval of atezolizumab treatment for PD-L1+ non-small cell lung cancer is a major step forward as a follow up treatment after surgery and chemotherapy.
When could it be available?
Since NHS England struck an early access deal with manufacturer Roche, it is expected to be used as soon as possible. Either February or March, 2022.
More than 850 patients in England are expected to be eligible for the drug in the first year, rising to more than 1,000 in the third year.
Dame June Raine, MHRA Chief Executive, said: “Through the MHRA’s membership of Project Orbis, an innovative programme coordinated by the US Food and Drug Administration (FDA) with other regulators across the world, we are working to ensure that patients receive earlier access to promising cancer treatments.”