Professor Ulrich Flögel, Heinrich Heine University Düsseldorf, explains how magnetic resonance techniques can be exploited to unveil crucial alterations in lipid metabolism and homeostasis
As has been pointed out previously, beyond imaging magnetic resonance techniques can also be employed to obtain important metabolic information in a non-invasive manner. In contrast to studies of intermediary metabolism, where – inter alia due to the low concentration of the metabolites of interest – the application of 13C-enriched substrates is almost indispensable (substrate usage), analysis of relevant aspects of lipid metabolism can be realised using the natural abundance of 13C (≈ 1%) in the long fatty acid chains. By means of a 13C surface coil, which is additionally inserted into the normal 1H resonator, 13C MR spectra can be acquired in acceptable time (≤ 20 min) providing a variety of information about the composition of the triglycerides deposited in lipid tissue.
As can be recognised in the spectum displayed, in particular carbons located at the end of the fatty acid chain (ω, ω-1, ω-2 and α, carboxylic, respectively) can be spectrally resolved in excellent quality. Furthermore, within the olefinic region distinct signals are detectable for mono- and polyunsaturated fatty acids (Δ and Δp), which can be used for determination of the relative amounts of saturated, mono- and polyunsaturated fatty acids (SAFA, MUFA, PUFA) in the triglyceride stores of the body.
Using the intensity ratio of the sum of all to the terminal fatty acid carbons, the average fatty acid chain length can additionally be calculated. Since the stored triglycerides reflect the biological fate of the ingested fatty acids over a prolonged period and, hence, constitute a kind of metabolic memory, this technique is ideally suited to investigate long-term effects of the nutrition on allover lipid homeostasis.
This is illustrated in the following when using this approach for studying the consequences of a dietary intervention for treatment of fatty acid oxidation disorders. In this context, very-long chain acyl-CoA dehydrogenase (VLCAD) deficiency is considered to be the most common inherited disorder of mitochondrial β-oxidation. Therapeutical approaches to prevent metabolic crisis during situations of increased energy demand include avoidance of fasting and a fat restricted and fat-modified diet, in which long-chain triglycerides (LCT) are replaced by medium-chain triglycerides (MCT).
In fact, medium-chain fatty acids are able to bypass the first step of β-oxidation catalyzed by VLCAD and are supposed to supply tissues and organs with the required energy, thus limiting metabolic derangements which may give rise for development of cardiomyopathy and skeletal myopathy. MCTs are considered as safe dietary intervention, however, long-term observations are still missing.
To investigate the impact of a prolonged MCT diet, VLCAD-deficient mice were used to mimic the human disease and exposed over 12 months, i.e. half of a mouse lifetime, to MCTs. 13C MR analysis of the dietary effects on fat composition revealed a dramatic drop in signals for polyunsaturated carbons – see red-border insert. Quantification of the spectra showed a PUFA content of only a fifth for the MCT diet as compared to normal diet (i.e. LCT).
Concomitantly, MUFAs were massively increased (see bar plot, left). Importanly, PUFAs have substantial cardioprotective properties through several mechanisms and are precursors for a variety of crucial metabolites such as eicosanoids including prostaglandins, leukotrienes, and thromboxanes with hormone-like activities. At the same time, MCT ingestion surprisingly had only a minor effect on the average fatty acid chain length as calculated from the ratio of signal intensities for terminal and nonterminal carbons.
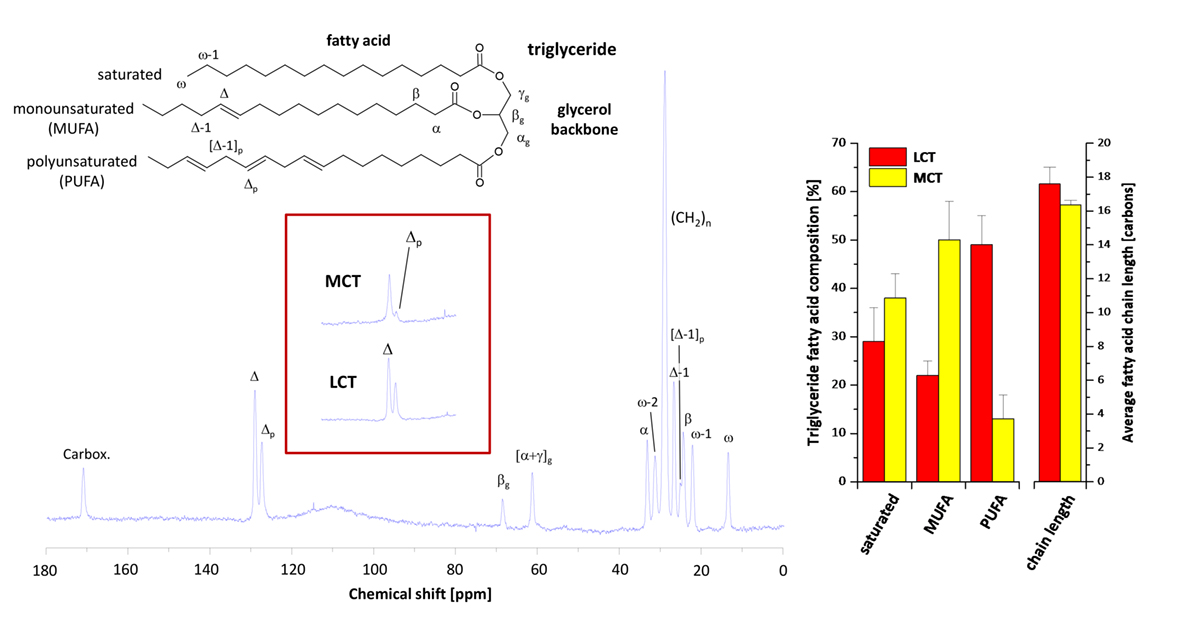
Although the MCT diet comprised mainly C-8 and C-10 triglycerides, the average fatty acid chain length in stored triglycerides was still found to be in the range of 16 to 17 carbons – even after an intake of this diet for one year – and quite in the same region as in mice exposed to a normal diet (see bar plot, right).
Obviously, ingested medium-chain fatty acids are somewhere in the body elongated to long-chain fatty acids – a process well known to occur in the liver. Because elongated fatty acids cannot be used for energy production in VLCAD-deficient mice, there are subsequently esterified and stored as LCTs. In the long run this results in a massive lipid accumulation in all organs (as shown in parallel by localized 1H MR spectroscopy) and constitutes a vicious cycle because of the continuously enhanced enrichment and turnover of medium-chain fatty acids in the liver. Further analysis indeed revealed that the metabolic alterations induced by the MCT diet led to severe liver damage in VLCAD-deficient mice with significant oxidative damage and steatohepatitis.
In summary, these exeriments demonstrate that MCT therapy of mice with a defect in ß-oxidation results in a severe phenotype similar to clinical metabolic syndrome. Importantly, the underlying biochemical reason – that spillover medium-chain fatty acids are not stored as MCTs in adipocytes, but are elongated to long-chain fatty acids thereby rendering the idea of the diet ‘to bridge the metabolic botteneck’ not only useless but actually detrimental (at least in this murine model) – have been identified by 13C MR spectroscopy; with this technique important metabolic indices are accessible which can not be obtained by any other non-invasive modality.
Thus, as a noninvasive strategy for surveillance of changes in body fat distribution and composition in treated patients, we argue for the use of such MR methods which could be readily transferred to clinical scanners.
*Please note: This is a commercial profile











