Thomas Daubon, Clotilde Billottet and Andreas Bikfalvi at the Angiogenesis and Tumor Microenvironment-INSERM U1029, Université Bordeaux provide insights into the mechanisms of primary brain tumour invasion
Glioblastomas (GBMs, WHO grade IV gliomas) are brain tumours derived from astrocytes or from oligodendrocytes (Kotliarova and Fine, 2012). GBMs are aggressive tumours that are characterised by a high proliferation rate, an abundant immature vascularisation, a necrotic core and infiltrative areas. The median survival of patients after diagnosis is very low, around 14 months despite surgery and radio/chemotherapy (Stupp et al., 2005). Anti-angiogenic therapy using Bevacizumab (Roche) led to a decrease in vascularisation and a shrinking of the tumour mass but overall survival was not significantly improved (Gatson et al., 2012; Weller and Yung, 2013).
Single-cell RNA sequencing identified different cell clusters within the tumour (Patel et al., 2014) and regional heterogeneity is also observed (Aum et al., 2014). Tumour evasion and relapse can originate from tumour cells infiltrating the normal brain parenchyma outside the subventricular zone or from the sub-ventricular zone. Spiteri et al (2019) performed whole-exome sequencing of multi-region samples from infiltrating tumour margins or the sub-ventricular zone. Phylogenetic reconstruction indicates residual tumour subclones may arise early and, thus, infiltration of the brain by tumour cells may arise at a very early stage.
Another line of investigation is to unravel the mechanisms that are operating at the level of the tumour cell. Recently, my laboratory has made some progress into this direction by focusing the attention on two molecular pathways including chemokines and extracellular matrix molecules (Fig. 1).
Chemokines are important mediators of cell signalling that operate both on normal cells and tumour cells and in the immune-cell compartment (for ref: Billottet et al, 2013). Among the chemokine receptors, CXCR3 mediate diverse biological functions and comes in two major isoforms the A and B isoforms. Promoting activities are generally associated with CXCR3A, while the B isoform is inhibitory. In a recent study (Boyé et al, 2017a), we found that ligand affinities and conformational changes are very different for the A and B form.
We have recently elucidated the role and mechanism of CXCR3A in GBM invasion (Boyé et al, 2017b). We demonstrated that agonist stimulation enhances in vitro cell migration and invasion in GBM cells. A major finding was that CXCR3A forms a complex with the trafficking receptor Lipoprotein-related receptor-1 (LRP1). We found that LRP1 enhances the trafficking of CXCR3A via a clathrin-dependent mechanism and retrograde recycling. Silencing of LRP1 leads to an increase in the magnitude of ligand-induced conformational change with CXCR3A focalised at the cell membrane, leading to sustained receptor activity and an increase in the migration. This was also clinically validated. Our study defines LRP1 as a new regulator of CXCR3 and indicates that targeting CXCR3A in GBM may constitute a promising strategy to halt tumour cell invasion.
The extracellular matrix (ECM) has morphogenic roles in tumours. Important ECM components are the matricellular proteins, called thrombospondins, a family composed of five members (THBS1-5) (Adams and Lawler 2011). THBS1, the most studied thrombospondin, may impact both, tumour cells and the microenvironment (Resovia et al 2014). It has been proposed that the effects of THBS1 on tumour cells are mediated indirectly through TGFβ activation via its type 1 domain, by mobilising its active form from the Latent Activating Protein (LAP) (Murphy-Ullrich et al, 2000). Another possible route for THBS1’s morphogenic effects is direct interaction with α6β1 or α4β1 integrins or cell surface receptors including CD36 and CD47 (Sid et al, 2004). How THBS1 is itself regulated, is also not known.
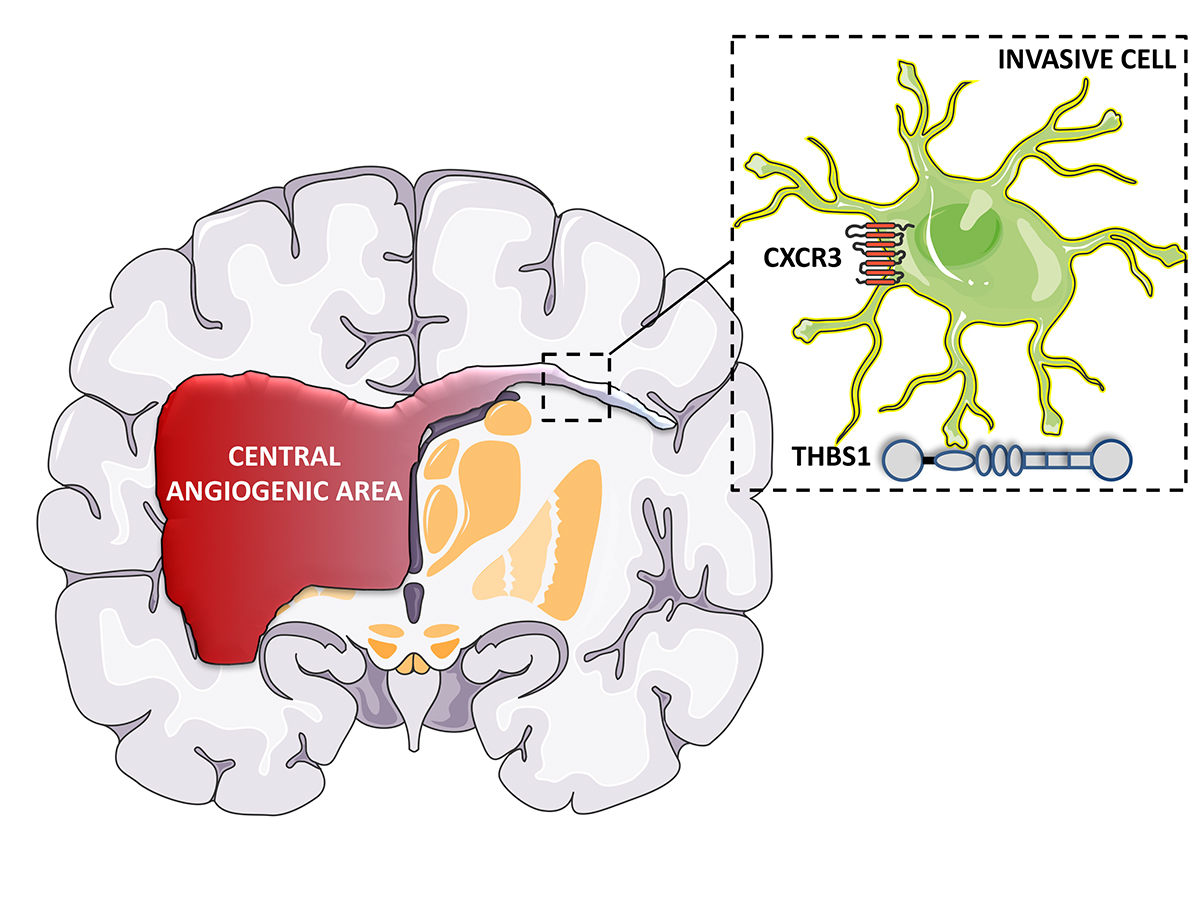
We recently elucidated the complex role of THSB1 in GBM invasion (Daubon et al. 2019). Global expression analysis revealed that THBS1 is up-regulated in GBMs and associated with a poor prognosis. We, furthermore, demonstrated that THBS1 did not activate TGFβ in GBM but that TGFβ1 induced the expression of THBS1 via SMAD3.
Furthermore, in vitro and in vivo GBM invasion is compromised when THBS1 is silenced in tumour cells. Thus, our data clearly shows that THBS1 is not only involved in the regulation of angiogenesis in GBM but also impacts the invasive behaviour of glioma cells by interacting with a molecule called CD47 expressed on the surface of GBM cells. RNA-sequencing after microdissection of central and peripheral tumour areas in a human PDX model demonstrated that THBS1 was the gene with the highest connectivity in the peripheral invasive tumour areas. Taken together, these data indicate that THBS1 plays important role in the infiltrative process in GBM.
GBM is one of the best-characterised tumours at the genetic level, but treatment options are still limited. Thus, additional mechanisms, such as microenvironmental cues, including cytokine- or matrix- dependent mechanisms must be explored for finding better treatment options to improve the clinical outcome. Our work constitutes a step towards this direction.
Please note: This is a commercial profile
References
Adams JC, Lawler J. Cold Spring Harb Perspect Biol. 2011;3:a009712.
Aum D et al. Neurosurg Focus. 2014; 37:E11.
Billottet C, Quemener C, Bikfalvi A. Biochim Biophys Acta. 2013;1836:287-
Boyé K et al. Sci Rep. 2017;7:10703.
Boyé K et al. Nat Commun. 2017;8:1571.
Daubon T et al, Nature Communications (in press).
Gatson NN, Chiocca EA, Kaur B. Neurosci Lett. 2012;527:62-
Hegi ME et al. N Engl J Med. 2005;352:997-
Kotliarova S, Fine HA. Cancer Cell. 2012; 21:710-710.e1.
Murphy-Ullrich JE, Poczatek M. Cytokine Growth Factor Rev. 2000 11:59-
Patel AP et al. Science. 2014;344:1396-
Resovia A, et al. Matrix Biology.2014, 37: 83-
Sid B et al. Crit Rev Oncol Hematol. 2004;49:245-
Spiteri I et al. Annals of Oncology, mdy506, https://doi.org/10.1093/annonc/mdy506
Weller M, Yung WK. Neuro Oncol. 2013; 15:971-
Andreas Bikfalvi
Professor
University Bordeaux and INSERM
Tel: +33 5 4000 8703