In a world first, the Medicines and Healthcare Products Regulatory Agency (MHRA) has approved trofolastat to detect cancerous lesions in men with prostate cancer
Prostate cancer is one of the most common cancers in men in the UK, with about one in eight men being diagnosed in their lifetime. It usually develops slowly over many years, often without symptoms, and its exact cause is unknown. As a result, many men may not realise they have it until it reaches a more advanced stage.
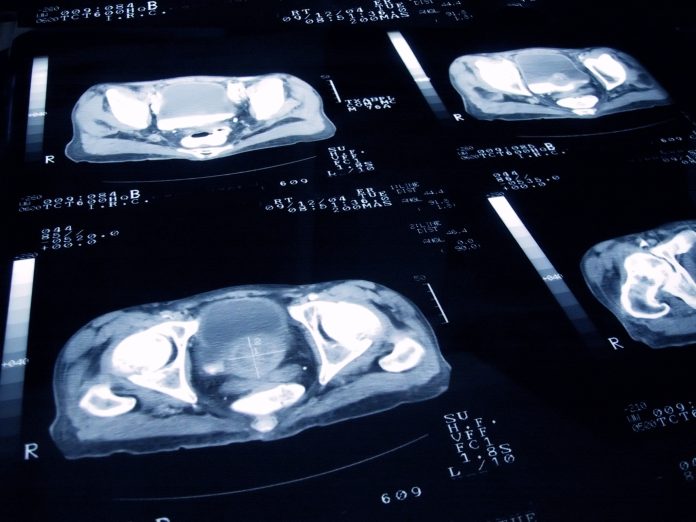
Diagnostic imaging is essential for detecting prostate cancer, especially in its early stages when symptoms may be absent. Techniques like MRI, ultrasound, and biopsy-guided imaging help identify cancerous areas, assess the disease’s extent, and guide treatment decisions. These tools are key in initial diagnosis and ongoing monitoring to ensure effective treatment.
Trofolastat (RoTecPSMA) is the first prostate-specific membrane antigen (PSMA)-targeting product authorised for use with technetium-99m to detect cancerous lesions in men with prostate cancer.
Supporting doctors with identifying cancerous areas
Trofolastat is typically combined with the radioactive tracer technetium-99m to form Technetium (99mTc) trofolastat, which is administered as a single injection. It then binds to a protein called PSMA found on prostate cancer cells, which helps doctors identify cancerous lesions during a medical imaging technique known as single photon emission computed tomography (SPECT).
Julian Beach, MHRA Interim Executive Director, Healthcare Quality and Access, said: “Keeping patients safe and ensuring access to high quality, safe and effective medical products are key priorities for the MHRA.
As the first PSMA-targeting diagnostic product approved with Technetium-99m, widely available in UK nuclear medicine facilities, this approval can expand access to prostate cancer imaging and support diagnostic pathways within the NHS.
The approval of this diagnostic product follows a rigorous assessment to ensure that it meets the required regulatory standards. As with all products, we will continue to monitor its safety and effectiveness.”
Trofolastat showed 94.2% sensitivity in identifying prostate cancer lesions.
The MHRA has approved the product for three clinical settings, including identifying how far high-risk prostate cancer has spread before treatment, detecting recurrence in patients with rising prostate-specific antigen (PSA) levels, and determining whether targeted therapies might be effective for metastatic prostate cancer patients.
Additionally, the approval follows a multi-centre, prospective study that involved 105 prostate cancer patients. Technetium (99mTc) trofolastat demonstrated 94.2% sensitivity in identifying prostate cancer lesions and an 83.3% specificity in confirming cancer-free areas.
The MHRA found that the most common side effect of Technetium (99mTc) trofolastat is a headache; however, the agency will continue to monitor the safety and effectiveness of the product.