Dr. Ludivine Herman, Project Leader at immunotherapy specialist Imcyse, walks us through Neuoromyelitis optica facts & trends, the challenges of current therapies and the potential of immunotherapies
It might start with weakness or numbness in the legs and arms. A shooting pain or tingling in the neck, back or abdomen. Sudden, blurry vision. The symptoms of Neuromyelitis optica (NMO) can vary and are often similar to those of other diseases such as MS, lupus and even some viral infections and cancers. Adding its rareness and the challenging underlying mechanisms of autoimmune diseases to the mix, approved therapies seemed out of reach for patients in desperate need of medical solutions. Yet, almost 100 years after NMO was first described, the approval of the first disease-modifying therapies has expanded the options available to patients and further candidates are on the horizon.
Similar but not the same – NMO not just a multiple sclerosis variant
For the longest time, NMO was considered an extreme variant of Multiple sclerosis (MS), one of the most prevalent disorders of the central nervous system (CNS), rather than a distinct disease. At first glance, this perception certainly makes sense: Similar to MS, NMO is an autoimmune disorder that damages the nervous system. With both conditions, instead of defending the body against bacteria and viruses, the patient’s immune system attacks nerve tissue causing inflammation. And people with either neurological condition may experience similar symptoms such as blurred vision, numbness or tingling in their legs. In reality, however, the two conditions are very different. Symptoms of Neuromyelitis optica are generally more severe with each relapse having significant potential to result in permanent damage. And unlike the most common forms of MS, NMO also is a progressive condition that worsens with each episode.
Having come a long way – the science behind Neuromyelitis optica
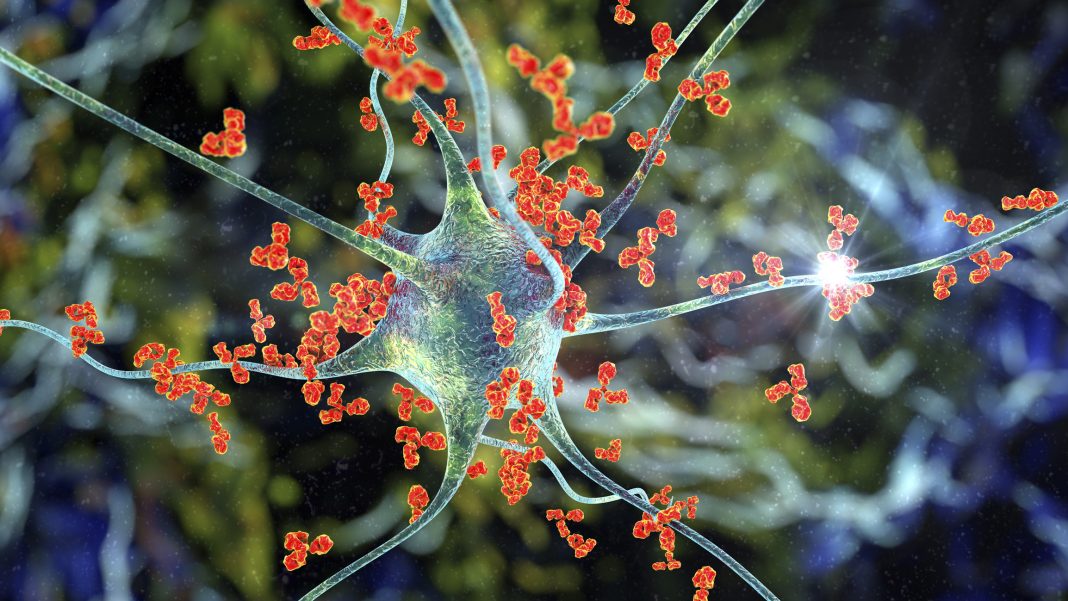
A great leap forward in understanding and distinguishing NMO as a separate disease entity occurred in 2004 when autoantibodies (termed NMO-IgG or AQP4-Ab) against the self-protein aquaporin 4 (AQP4) were identified.
This discovery encouraged interest in the disease, and it is now common consensus that, contrary to MS, where your immune system attacks the myelin sheaths or the cells that produce and maintain it, NMO antibodies recognise AQP4, a water channel protein expressed on Astrocytes, that regulates how water moves in and out of cells. These nerve-cell caretakers live in the brain and spinal cord, and depending on where the attack takes place, different symptoms will emerge, such as sudden vision loss or partial paralysis.
The hurdles of getting the right diagnosis
As with many other autoimmune diseases, diagnosing Neuromyelitis optica often requires ruling out other possible conditions with similar symptoms. Due to the similarities with MS – especially in its early stages – and unfamiliarity with NMO, misdiagnosis can often occur. As the treatments for both conditions can differ significantly, getting an accurate diagnosis is extremely important; reports suggest that some therapies for MS are ineffective for or can even worsen NMO. In 2015, diagnostic criteria were established by an international panel to facilitate earlier and more accurate diagnosis. An important advance was the generation of a cell-based antibody test which more accurately recognises the antibodies involved in the disease. This blood test detects the presence or absence of serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG), which are present in 73–90% of patients.
From zero to three approved therapies in just a decade
As disability in NMO accumulates as a result of inflammatory damage, both treatment and prevention are needed for improved outcomes. Traditionally, various immunosuppressive drugs and regimes have been a cornerstone in the treatment of Neuromyelitis optica as, until recently, targeted, disease-modifying agents were unavailable.
Fortunately, the recent improvements in our understanding of the underlying immunopathological mechanisms facilitated the development of new therapies. After more than 100 years since NMO was first described, three new (monoclonal) antibody therapies that provide some protection against relapses have become available to patients.
Each of these antibodies targets a specific immune cell or mediator and suppresses that part of the immune response. And while patients do benefit greatly from these therapies, they still impact many aspects of the immune system, potentially making patients more susceptible to infections and other side effects.
Developing the next-generation specific immunotherapies
To provide even better therapeutic options and ultimately find a cure, several companies and academic groups are developing new approaches which aim to target the immune system in a more specific way in order to decrease the side effects and develop more effective therapies. By only blocking the disease pathway, these approaches aim to selectively suppress the specific malfunctioning part of the immune system while allowing the rest to continue functioning normally, i.e. allowing for control of infectious diseases and cancers.
Forging new ways – committed to transforming the treatment of autoimmune diseases
One such specific immunotherapy that targets the underlying cause of the disease is currently being developed by Belgian immunotherapy specialist Imcyse. Based on the Company’s proprietary platform, Imcyse develops synthetic peptides – ImotopesTM – that block defective immune responses causing diseases such as NMO and reset the immune system.
In a nutshell, ImotopesTM induce cytolytic CD4 T cells that can, in a very specific manner, kill other immune cells involved in the disease. As ImotopesTM are antigen-specific and do not cause a generalized immune suppression, i.e. do not affect other functions of the immune system, and so far fewer side effects have been observed, resulting in a more favorable safety profile. ImotopesTM have a long lasting effect by stimulating Immune Memory like a vaccine. This means once the beneficial effect of ImotopeTM is established, it lasts for a long time and, hopefully, boosters can maintain the effect. The aim of this approach is to intervene early enough in the disease progression so the aberrant immune response is blocked and the immune system is recalibrated to stop, in the case of NMO, the destruction of astrocytes, preserving the patients’ sight. A more detailed description of Imcyse’s technology is available in a previous article.
What comes next for our patient therapy
At Imcyse, we will continue to drive the development of our pioneering approach so patients around the world can one day benefit from our therapy. Having established proof of concept in several indications and having completed a first clinical trial in lead indication Type 1 diabetes with promising results, Imcyse is currently preparing the first clinical trial to test an NMO-specific ImotopeTM with the support of the expertise of the Guthy-Jackson Charitable Foundation. A submission to regulatory authorities is anticipated for Q4 2023.
And while autoimmune diseases currently remain incurable –new targeted therapies such as Imcyse’s approach provide hope for patients around the world that one day, there will be a cure.
For more information on Neuromyelitis optica please visit: https://guthyjacksonfoundation.org/

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.