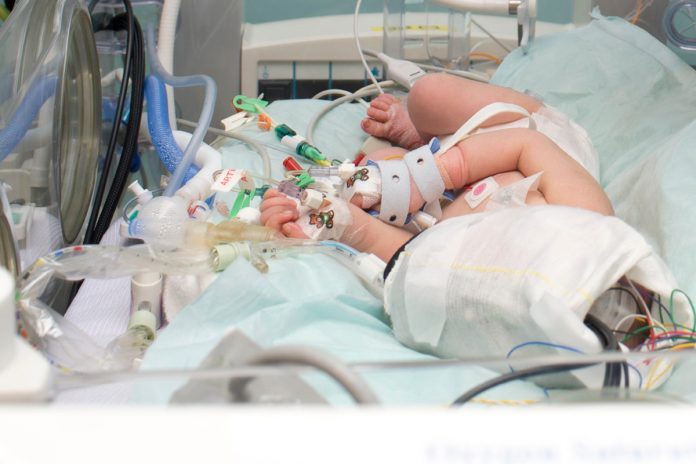
A new direction in the monitoring of brain injury in babies, pioneered by a team of physicists, engineers and doctors in University College London (UCL) and University College London Hospitals (UCLH)
In this profile, Dr Ilias Tachtsidis, Wellcome Trust Senior Fellow, Reader in Biomedical Engineering at UCL and Dr Subhabrata Mitra, Consultant Neonatologist at UCLH introduce MetaboLight, explain how physicists, engineers from the Department of Medical Physics and Biomedical Engineering at UCL, working together with medical doctors from the Neonatal Unit at UCLH have demonstrated innovation concerning the monitoring of newborn brain injury, with the development of technologies and instruments that allow early diagnostics of brain injury severity.
Early insults to brain development can result in significant long-term morbidity and mortality. Unfortunately, many infants are born in difficult conditions, with a lack of blood and oxygen supply to brain around the time of birth. Brain function becomes deranged in this group of infants and they develop hypoxic ischaemic encephalopathy (HIE). In developed countries, 2-3 infants per 1000 live births suffer from this condition and unfortunately, this incidence can be up to 10 times higher in countries of sub-Saharan Africa and Asia.
Therapeutic hypothermia (total body cooling) is the only clinical treatment option that has been shown to improve outcome (death or disability) following this condition. It has become a standard of care from 2010 in the UK. During this treatment, the infant is cooled for 72 hours and then gradually warmed back to the normal temperature, over a period of 14 hours.
The clinical challenge
Following HIE, significant changes are noted in brain metabolism. The energy state of the brain changes through stages of energy depletion and apparent recovery, before going through final depletion, if not acted on early. These stages are called primary and secondary energy failure. Most of this neuro-metabolic cascade of changes occur within the powerhouse of the cell, mitochondria.
In addition to HIE, other neurological conditions like neonatal stroke and seizures are also known to cause disturbances in brain metabolism and oxygenation. So, it is important to focus on attempts to monitor mitochondrial metabolism, along with brain oxygenation continuously at baby’s cotside to understand the degree of injury and response to treatment in different neonatal neurological conditions.
There is an unmet demand for developing an early cotside biomarker – that will give us the information regarding changes in the pathophysiological state of the brain function during and following treatment, and it will relate to future outcome.
The engineering challenges
Once the infant becomes stable after the cooling treatment, an MRI (Magnetic resonance imaging) and an MRS (magnetic resonance spectroscopy) of the brain is performed (normally between day 5-8 of life) to assess the degree of injury and to predict the future neurodevelopmental outcome.
MRS derived brain Lac/NAA (lactate/ n-acetyl aspartate) peak area ratio is a robust biomarker of the outcome. MRI and MRS are expensive instruments that require dedicated staff, can only be used several days after the injury, when the infant becomes stable and because they require the infant to be moved from the intensive care unit, they carry significant risk in sicker infants.
Given the complexity of the other monitoring in place for these infants, any new monitoring needs to be easily applicable from day one following birth and can be monitored by clinical staff.
Our technology and Measurements
To answer these challenges, we employ an optical technique using harmless near-infrared light that can probe the brain tissue. The technology is known as near-infrared spectroscopy (NIRS) and has been used to monitor accurately the changes in brain haemodynamics and oxygenation, using simple optical probes placed over the surface of the scalp.
NIRS instruments transmit a couple of different wavelengths (colours) of near-infrared light to the surface of the head; the reflected light from the brain tissue has been attenuated by the haemoglobins in the blood vessels that carry oxygen. The amount of oxygen carried by the haemoglobins will affect the colour of the blood in the brain tissue; that is why arterial blood is red (fully oxygenated) and venous blood is blue/purple (less oxygenated). NIRS can quantify this colour and hence measure oxygenation.
But we had to go further and develop solutions that allow us to monitor the mitochondrial function. To achieve this, we have developed and used broadband NIRS technology, which means that instead of using only two or three different colours of near-infrared light we use hundreds.
This allows us to monitor many optical signatures, besides the haemoglobins within the brain tissue. One unique optical signature comes from within cytochrome-c-oxidase, an enzyme inside the mitochondria. This enzyme is one of many responsible in converting glucose and oxygen to energy; by facilitating this process it changes colour and means that we can detect it with our novel broadband NIRS technology. We now have technology that can continuously measure brain oxygenation and mitochondrial metabolism using harmless near-infrared light.
Broadband NIRS instrument
We have developed instruments that use white light sources, similar to the headlights in cars to transmit many colours of near-infrared light through optical fibres attached to the head of the infant. We collected the reflected light from the head with another fibre optic cable, that is connected to a detection unit that is called a spectrometer.
A spectrometer is an instrument often used in biology and astronomy, that is capable of splitting the light to its many colours. It then uses sensitive digital cameras to measure the changes in light intensity for every colour. As oxygenation and metabolism changes, the measured colour intensities will change. The broadband NIRS is made of easily sourced and relatively cheap components, non-invasive, harmless, highly portable and can be positioned by the cotside in the neonatal intensive care unit to measure the changes in brain tissue oxygenation and mitochondrial metabolism, continuously in real time from early on after a suspected brain injury.
Our current research results
Using our novel broadband NIRS instrument, we have now monitored nearly one hundred babies suffering from different neurological conditions including HIE, seizures and neonatal stroke. We have demonstrated that using metabolic biomarkers, sicker infants can be identified early after HIE, and how the injured brain metabolism responds differently to available oxygen delivery following injury. We have also identified new insights in the brain metabolic changes during neonatal seizures and following the neonatal stroke.
These results have brought us a closer to a robust biomarker of neonatal brain injury. This work is part of our feasibility study supported by the Wellcome Trust and further information can be found in our public engagement and dissemination web platform www.metabolight.org.
Please note: this is a commercial profile
Dr Ilias Tachtsidis
Wellcome Trust Senior Fellow and Reader in Biomedical Engineering
University College London
www.twitter.com/IliasTachtsidis
Dr Subhabrata Mitra
Consultant Neonatologist
University College London Hospitals