Dr Frank Wuest, Professor at Department of Oncology – University of Alberta, sheds light on the imaging biomarkers and the Alberta Radiopharmaceutical Collaboration in this special oncology focus
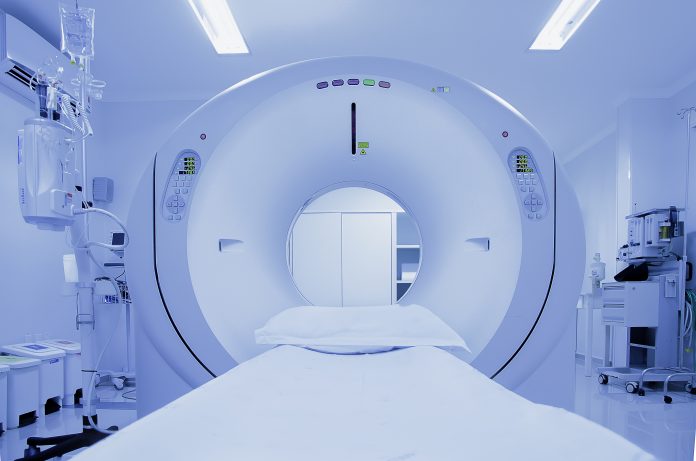
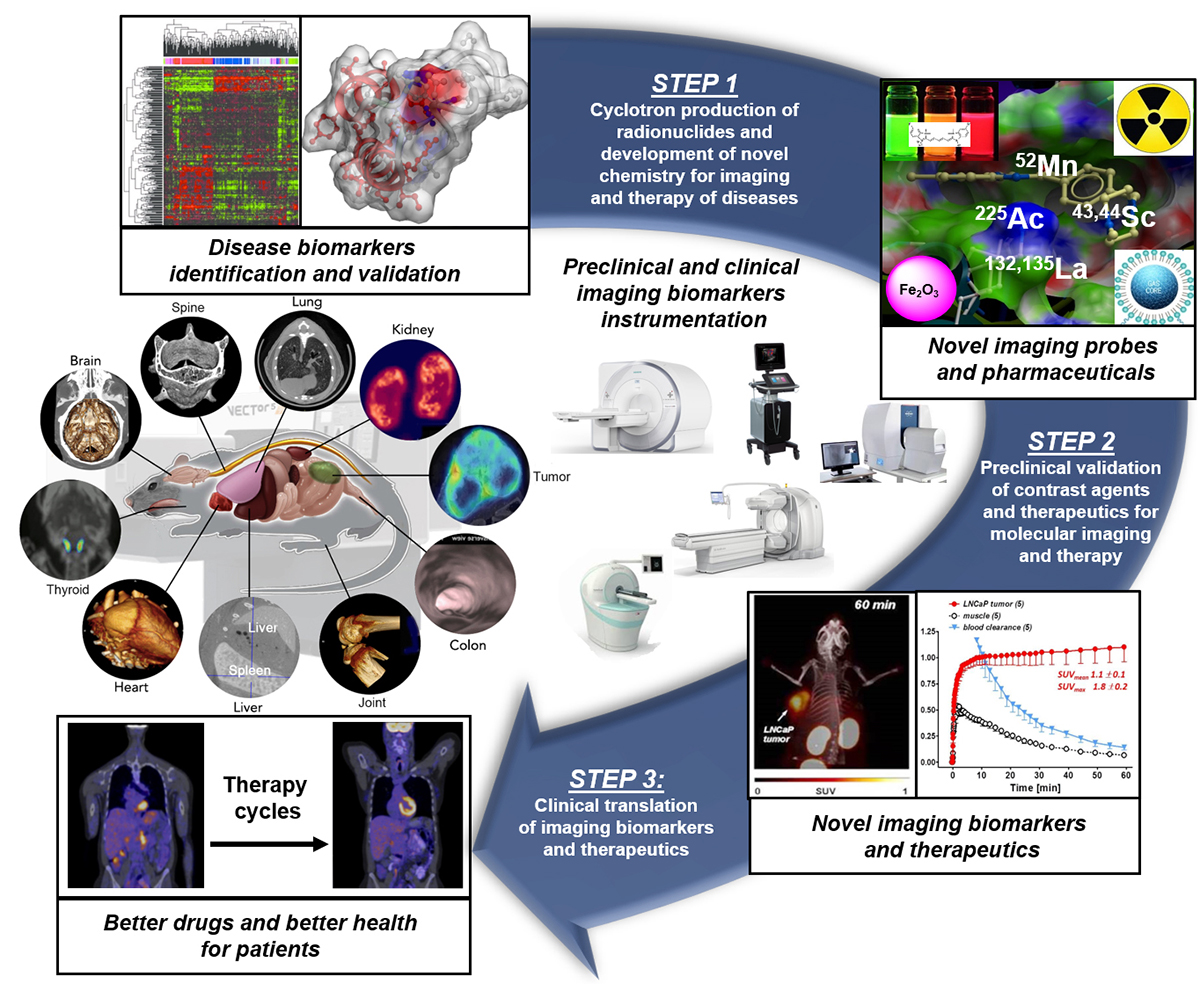
The molecular processes of life and diseases can be studied and visualised at various levels of resolution in humans and animals with imaging biomarkers. Imaging biomarkers are defined as a subset of anatomical, physiological or molecular biomarkers that manifest themselves via imaging means, including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), single photon-emission tomography (SPECT) and positron emission tomography (PET). Imaging biomarkers are pivotal components of the multi-step precision medicine translational research path that ultimately provides far better patient outcomes, with far less morbidity and mortality. Moreover, imaging biomarkers are also linked to recent advances in drug discovery and drug evaluation (Figure 1).
Imaging biomarkers in concert with pharmacokinetic/pharmacodynamic (PK/PD) modeling can non-invasively measure the distribution of contrast agents and drugs in both space and time, allowing us to assess biological processes and decipher how – across the entire body – disease-associated biomolecules contribute. Today, medical images are crucial tools to evaluate biomarkers for disease diagnosis plus staging and predicting the response to therapeutics. For example, in clinical cancer research, we are beginning to use imaging biomarkers to diagnose, stage, and characterise the phenotype of tumours, including clonal variations, using multimodal PET/CT, PET/MRI, and SPECT/CT imaging.
Cancers are increasingly classified by their molecular phenotypes to provide critical information for more effective treatment options, and their site of origin is becoming less relevant. Likewise, across many other pathologies, the molecular nature of the disease and how best to treat it will be increasingly determined by molecular pathology, including imaging biomarkers and PK/PD modeling techniques. The complexity and heterogeneity of the underlying disease biology in cancer, cardiovascular, neurological, metabolic and inflammatory diseases require an individualised biomarker-based patient assessment in combination with machine learning and artificial intelligence data evaluation tools to develop and guide optimal personalised therapies and prevention strategies, the cornerstones of the precision medicine concept. With such tools, we can design and guide optimal personalised treatments and prevention strategies as the cornerstones of precision medicine. Today, images are no longer seen as a mere anatomical depiction of disease presence or absence but instead used as imaging biomarker roadmaps to decipher the molecular basis of diseases and define new predictive, prognostic, and therapeutic instruments that improve disease outcomes.
Imaging biomarker roadmaps depend on innovative imaging agents and therapeutics that target molecular pathways integral to a specific disease. Generating these agents and validating their diagnostic and therapeutic potential for clinical translation requires unique expertise, established clinical cohorts, and substantial time and economic investment.
Pre-clinical and clinical nuclear medicine research
Since its inception, the medical isotope production, imaging biomarkers, and radiotherapy program of the University of Alberta (UofA) and Cross Cancer Institute (CCI) have become a Canadian flagship in pre-clinical and clinical nuclear medicine research and patient care. The imaging biomarkers and nuclear medicine research program at the UofA and CCI have become internationally recognised and stimulated the creation of significant local, national, and international collaborations and partnerships with academia, industry, and the community. The program led to the enrolment of over 8,000 cancer patients in 23 investigator-initiated clinical trials and into 38 phase 1, 2, and 3 industry-sponsored clinical trials. The program has also become a significant research resource for the broader cancer clinical trial community in Alberta but has also supported clinical trials in patients with diseases other than cancer. In September 2019, the provincial healthcare provider Alberta Health Services (AHS) and the UofA founded the Alberta Radiopharmaceutical Collaboration (ARC) to coordinate and integrate imaging biomarkers clinical research capacities in Alberta. The ARC provides an exceptional environment for world-class research, innovation, and training of scientists, clinicians and technologists in radiopharmaceutical sciences and imaging biomarkers. The result is a better standard of patient care with radiopharmaceuticals in the province of Alberta and Canada. Moreover, both AHS and the UofA have identified precision medicine and imaging biomarkers as institutional priorities.
The strength and strategic importance of the imaging biomarkers program at the UofA have been a consistent and robust foundation for high-quality research and innovation in radiopharmaceutical sciences and nuclear medicine. The UofA imaging biomarkers research program is on the national and international forefront for the development and validation of innovative radiopharmaceuticals as candidates for clinical translation and initiation of first-in-human studies. Following the imaging biomarkers translational research path, the ARC has identified (1) the cyclotron production and validation of innovative radionuclides; (2) the development of novel chemistries for imaging and therapy of diseases; (3) the validation of novel radiopharmaceuticals; and (4) the rapid and safe clinical translation of innovative radiopharmaceuticals through rigorous regulatory processes as priority clinical imaging biomarker research directions with high knowledge transfer capacity to enhance patient care and improve patient outcomes.
Facilitate introduction of novel radiopharmaceuticals
The mission of the ARC research program is to facilitate the rapid and safe clinical introduction of novel radiopharmaceuticals that will significantly enhance patient care in an economic environment that is particularly challenging for niche products such as radiopharmaceuticals. The integration of a robust regulatory core will harmonise and bring rigour to the spectrum from pre-clinical development to manufacturing to clinical trial conduct with radiopharmaceuticals. ARC research priorities reflect the core competencies in radiopharmaceutical sciences, imaging biomarkers, and nuclear medicine in the province of Alberta and Canada.
Please note: This is a commercial profile