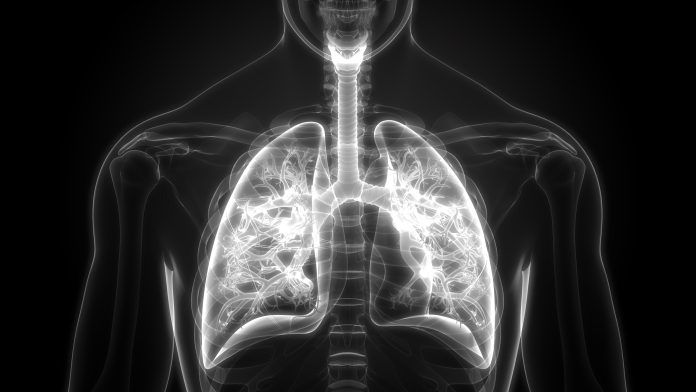
New estimates put one in 3,000 people at risk of a defective gene that increases their risk of a punctured lung
The gene, FLCN, is linked to a condition called Birt-Hogg-Dubé syndrome, which causes symptoms such as benign skin tumours, lung cysts, and a lifetime risk of a punctured lung. Previous research indicated that one in 200,000 people were at risk of carrying the faulty gene, but new research suggests this figure is closer to one in 3,000.
“If an individual has Birt-Hogg-Dubé syndrome, then it’s very important that we’re able to diagnose it, because they and their family members may also be at risk of kidney cancer”, commented Stefan Marciniak.
Analysing data from more than 550,000 people
Researchers from the University of Cambridge examined data from the UK Biobank, the 100,000 Genomes Project, and East London Genes & Health – three large genomic datasets encompassing more than 550,000 people.
They discovered that one in 2,710 and one in 4,190 individuals carry the particular FLCN variant underlying Birt-Hogg-Dubé syndrome. Patients with a diagnosis of Birt-Hogg-Dubé syndrome have a lifetime risk of puncture lung of 37%, but in the broader cohort of carriers of the genetic mutation, this was lower at 28%. Surprisingly, patients with Birt-Hogg-Dubé syndrome have a 32% chance of developing kidney cancer; in the broader cohort, this was only 1%.
New insights into punctured lungs
Punctured lung, known as pneumothorax, is caused by an air leak in the lung, leading to painful lung deflation and shortness of breath. The punctured lung can occur due to other conditions; for example, around one in 200 tall, thin young men in their teens and early twenties will experience a punctured lung, which typically resolves itself.
Professor Marciniak is a researcher at the University of Cambridge and an honorary consultant at Cambridge University Hospitals and Royal Papworth Hospital. He co-leads the UK’s first Familial Pneumothorax Rare Disease Collaborative Network with Professor Kevin Blyth, aiming to improve care, treatment, and research for patients with inherited familial pneumothorax.
Professor Marciniak said: “If an individual has Birt-Hogg-Dubé syndrome, then it’s very important that we’re able to diagnose it, because they and their family members may also be at risk of kidney cancer.
“The good news is that the punctured lung usually happens 10 to 20 years before the individual shows symptoms of kidney cancer, so we can keep an eye on them, screen them every year, and if we see the tumour, it should still be early enough to cure it.”
Professor Marciniak said he was surprised to discover that the risk of kidney cancer was so much lower in carriers of the faulty FLCN gene who have not been diagnosed with Birt-Hogg-Dubé syndrome.
“Even though we’ve always thought of Birt-Hogg-Dubé syndrome as being caused by a single faulty gene, there’s something else going on,” Professor Marciniak said. “The Birt-Hogg-Dubé patients that we’ve been caring for and studying for the past couple of decades are not representative of when this gene is broken in the wider population. There must be something else about their genetic background interacting with the gene to cause the additional symptoms.”
The finding raised the question of whether, if an individual is found to have a faulty FLCN gene, they should be offered screening for kidney cancer. However, Professor Marciniak does not believe this will be necessary.
“With the increasing use of genetic testing, we will undoubtedly find more people with these mutations,” he said, “but unless we see the other tell-tale signs of Birt-Hogg-Dubé syndrome, our study shows there’s no reason to believe they’ll have the same elevated cancer risk.”