Prof Masahiro Kamitani from the Department of Chemistry at Kitasato University, Japan, introduces recent advances in the development of catalysts for organic synthesis and iron catalysts as alternatives for precious metal catalysts
Transition metal catalysts are indispensable tools for efficient and economic chemical reactions for producing chemical products and modifying chemicals in products (Figure 1). A three-way catalyst promotes the conversion of pollutants in exhaust gas to chemicals with less environmental burden before emission; this is an example of catalysts working on chemical products. The chemical reactions to produce organic molecules such as medicinal drugs also depend on transition metal catalysis. A. Suzuki, E. Negishi, and R. F. Heck were awarded the Nobel Prize in 2010 “for palladium-catalyzed cross couplings in organic synthesis.”
Precious metal catalysts such as palladium are considerably effective and extensively used for organic synthesis, especially in medicinal chemistry and the manufacturing of drugs. However, unlike catalysts for use in automobile exhaust, drugs are consumed orally. Therefore, toxic impurities such as heavy or precious metals, like palladium, platinum, and iridium, used for catalysis in medicinal drugs are closely monitored and must be removed before packaging. Ensuring satisfactory purity for safety requires considerable time, effort and energy. Considering these points of view, catalysts that are economic and less toxic are being actively investigated in industry and academia.

Organocatalysts
One promising alternative of precious metal catalysts is “organocatalysts”, which have gained increasing attention since D. W. C. MacMillan and B. List were awarded the Novel Prize in 2021 for their contribution in this field. Organocatalysts primarily comprise of non-metal elements such as carbon, nitrogen and hydrogen, along with minor amounts of some other elements. When organocatalysts are used instead of precious metals catalysts, the problem of residual metals after the chemical reaction during drug manufacturing is resolved. There are still few alternative reactions; however, organocatalysts are advantageous for practical use over transition metal catalysts, and further development is underway.
Iron catalysts
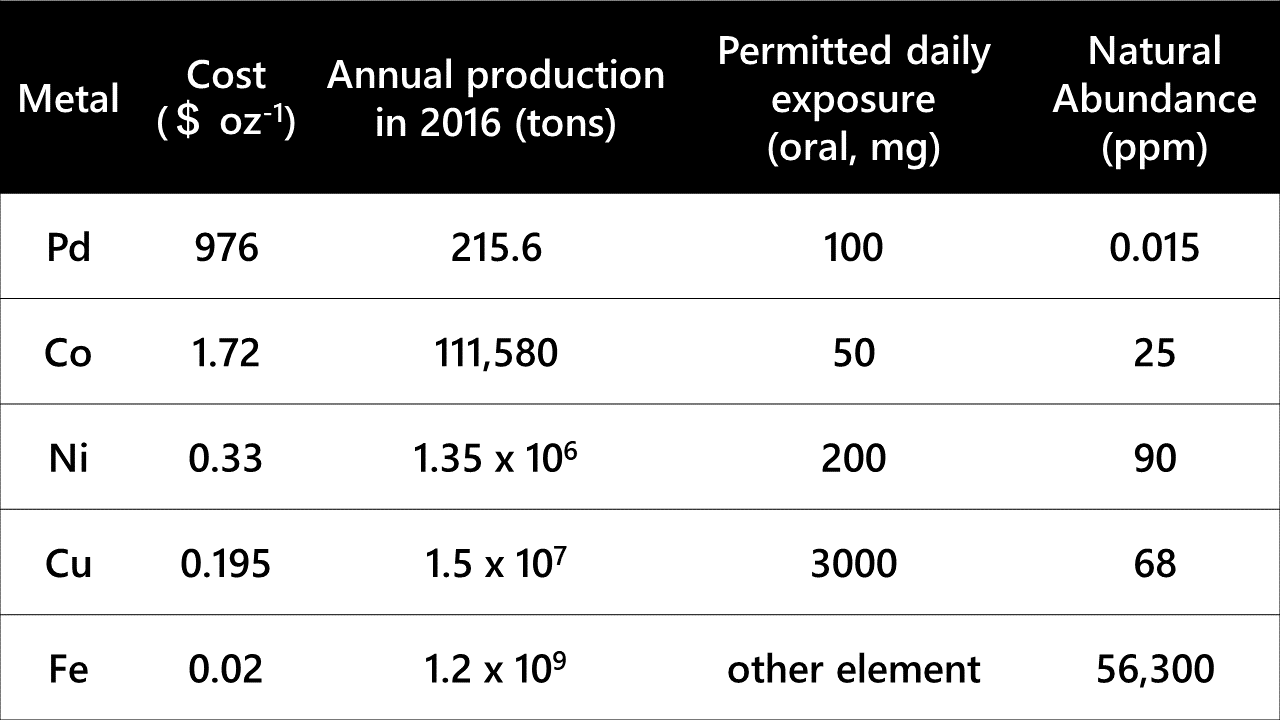
Another alternative is the use of iron, which is the most abundant transition metal on the earth as well as in the human body. Owing to its ubiquitous nature, iron is less toxic to organisms and environmentally benign (Table 1, M. C. Bryan et. al., Green Chem., 2018, 20, 5082). The low toxicity of iron is most important for the organic synthesis of medicinal drugs. In addition, using iron catalysts is advantageous over using precious metals because of their cost efficiency and abundance. Iron catalysts for organic synthesis have been actively investigated for a few decades now, and a variety of reactions have been achieved using iron catalysts. However, most of them have exhibited much lower performance than those of precious metal catalysts.
Iron superior to precious metals
Our group recently developed a series of iron catalysts equipped with both stability and activity for practical use in industry (M. Kamitani, Chem. Commun. 2021, 57, 13246.). These catalysts have exhibited excellent performances comparable to platinum catalysts used in industry for the hydrosilylation of alkenes for producing silicone materials.
Iron catalysts also work well for organic synthesis, for example, in the C-H borylation of arenes, which is an atom economic reaction to produce organoboron compounds. Although iridium catalysts, [Ir(COD)X]2 X = Cl, OMe, have been widely used for the above chemical processes, these precious metal catalysts have to be stored under cold and nitrogen atmosphere to avoid decomposition by exposure to air, moisture or heat. Iron catalysts developed in our research can be stored in an air atmosphere because they are not sensitive to air, moisture or heat. This versatile iron catalyst is the first step toward its commercialisation, and we continue to develop further applications of iron catalysts.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.