The term “forever chemicals” has become part of the lexicon in lay science. This reflects the growing evidence that exposure to perfluoroalkyl substances (PFAS), a class of synthetic chemicals widely detected in our natural environment, our foods, and our bodies, is associated with adverse human health effects and harm to ecosystems
However, while PFAS has become a predominant environmental health concern, they are not the only class of forever chemicals that pose a health risk. Rather, they belong to a diverse group of chemicals that share the property of being remarkably stable in the environment. Other persistent organic pollutants (POPs), which is the scientific term for forever chemicals, include polychlorinated biphenylsl (PCBs), dioxins and organochlorine pesticides like DDT (Table 1).
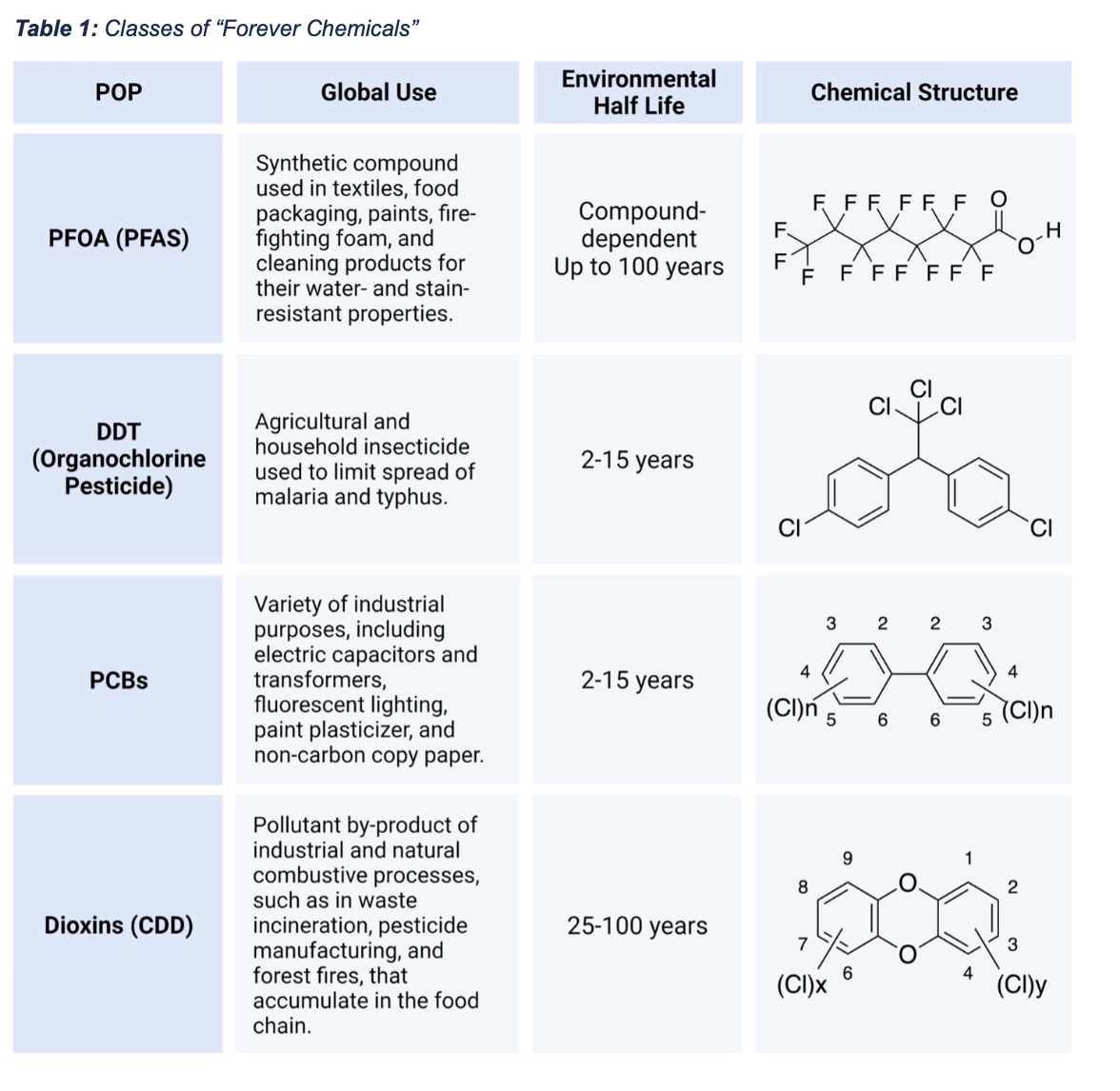
The problem of forever chemicals first came to public attention in the mid-20th century with Rachel Carson’s pioneering 1962 publication, “Silent Spring,” which chronicled the adverse impacts of DDT on wildlife and, by implication, humans. However, regulation of forever chemicals lagged, and it was only in 1979, after nearly half a century of high-volume production, that PCB manufacturing was banned in the U.S. In 2001, the United Nations’ Stockholm Convention on POPs took steps to monitor and regulate forever chemicals globally, identifying a “Dirty Dozen” for restriction and cleanup. PFAS were recently added to this list.
PFAS are a diverse group of human-made compounds that include thousands of chemical species. The Organisation for Economic Cooperation and Development (OECD) recognizes almost 5,000 distinct PFAS, while the United States Environmental Protection Agency (USEPA) lists 14,735 unique PFAS. As a chemical class, PFAS are defined structurally as chains of carbon-fluorine bonds. The carbon-fluorine bond confers heat resistance, surfactant properties, and chemical stability. The first PFAS chemical, polytetrafluoroethylene (PTFE), was synthesized by DuPont chemist Roy J. Plunkett in the 1940s. DuPont quickly recognized the commercial potential of Plunkett’s discovery and began marketing PTFE under the name “Teflon”. PTFE was heralded as a miracle of modern chemistry because of its extreme chemical stability and ability to repel both oil and water. Over the next several decades, many PFAS derivatives were synthesized, including perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), which are now known as “legacy PFAS”, and more recently, “GenX” PFAS. Many have been used in countless industrial and consumer products.
While PFAS were a huge commercial success, DuPont glossed over alarming company generated evidence that PFAS interact with and alter biological systems. The Environmental Working Group, an environmental advocacy organization, unearthed internal DuPont documents dating back to shortly after the discovery of PFAS that painted a damning portrait of manufacturer negligence. Decades of DuPont research described evidence of PFAS toxicity that was withheld from the public. In parallel, studies by scientists not associated with DuPont revealed widespread environmental contamination with PFAS. A 2003-2004 United States National Health and Nutrition Examination Survey found high levels of PFAS in >98% of serum samples obtained from U.S. citizens. Such findings prompted further examination of PFAS in biological and environmental matrices, which revealed extensive PFAS contamination of drinking water sources across Europe, Asia, and the Americas, and in ecosystems as isolated as the Arctic.
Environmental fate
As a class, PFAS are remarkably stable in the environment with decades-long environmental half-lives (Table 1). This is in part because the carbon-fluorine backbone of PFAS, which is one of the most stable chemical bonds, confers resistance to biodegradation (breakdown by living organisms), photooxidation and photolysis (degradation by ultraviolet radiation), and hydrolysis (water- associated molecular cleavage). While some GenX PFAS, notably the fluorinated telomer alcohols (FTOH), can be broken down in the environment, they often are degraded into environmentally persistent legacy PFAS.
The predominant sources of PFAS contamination are manufacturing and industrial sites, military installations, airports, and landfills (Figure 1). Conventional wastewater treatment does not remove PFAS, resulting in numerous contamination incidents tied to industrial effluents, most recently in the Cape Fear River Basin in North Carolina. U.S. Military installations and airports are also major sources of PFAS contamination due in large part to the use of PFAS-containing firefighting foams that have led to near-ubiquitous PFAS contamination of the environment adjacent to such facilities. PFAS-containing consumer items buried in landfills release PFAS into leachate, which seeps into groundwater and soil.
Once in the environment, PFAS behave similarly to other POPs, spreading widely through water and air to locations distant from their origin. For many PFAS, groundwater and surface waters are major environmental sinks because the high-water solubility of PFAS enables these compounds to move easily into aqueous media, while their low volatility limits evaporation. As a result, PFAS tend to accumulate in water, including drinking water sources, which has become a critical public health concern. PFAS pollution of marine ecosystems is also increasing. While water has historically been considered the primary environmental reservoir for PFAS, emerging research indicates significant contamination of soils near sites of PFAS emission, with orders-of-magnitude greater PFAS concentrations in contaminated soils compared to typical groundwater concentrations. Such data suggest that PFAS-containing soils may act as sustained sources of PFAS in waters and vegetation, including crops.
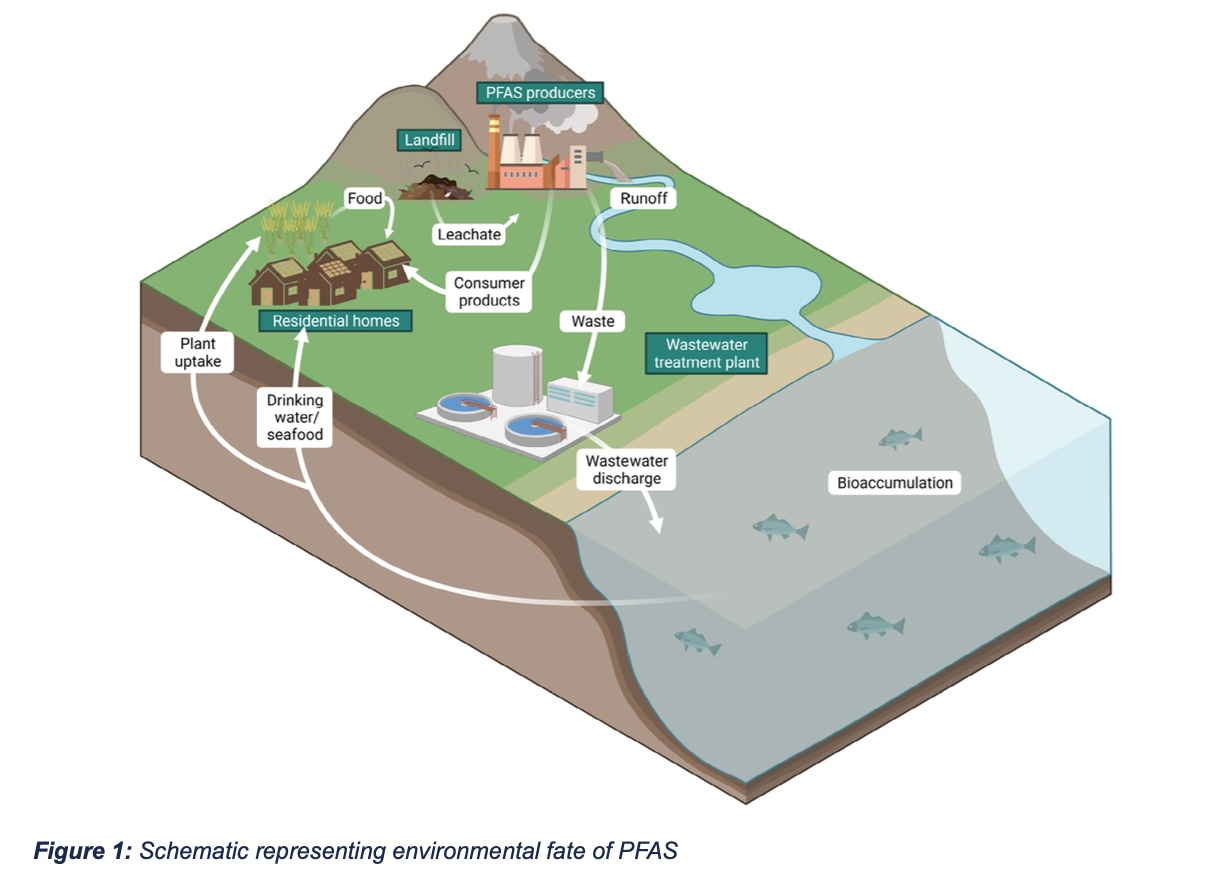
While PFAS predominantly move through the environment via water, PFAS can undergo long-range atmospheric transport. Most PFAS do not volatilize. However, long-range atmospheric transport has been observed following the association of non-volatile PFAS with airborne organic matter and dust. Such interactions have been identified in atmospheric discharges from PFAS manufacturing facilities and aerosols produced at the water-atmosphere interface of PFAS- contaminated water sources, enabling the atmospheric spread of these non-volatile chemicals. Some PFAS species, such as FTOHs, will readily volatilize and thus can be transported long distances by air currents. Volatile FTOHs have an estimated 20-day atmospheric half-life, a sufficient residence time to enable wide distribution from emission sites. It is likely that atmospheric transport of both non-volatile and volatile PFAS is similar to that of other POPs, with alternating cycles of volatilization and deposition, resulting in accumulation of these species in cooler arctic environments through a process known as “grasshopping.”
PFAS toxicity
PFAS have been detected in nearly every biome and environmental matrix, and a substantial body of research describes the detection of PFAS in humans, companion animals, production animals, and hundreds of wildlife species. PFAS contamination is particularly widespread in marine ecosystems, with perfluorinated species detected in organisms at every food chain level. Importantly, PFAS concentrations in marine wildlife are consistently higher than ambient water, and the highest levels of PFAS are found in apex predator species, indicating that PFAS are biomagnified or concentrated up the food chain. Similarly, PFAS body burdens in human populations often exceed PFAS concentrations in contaminated drinking water and foods.
PFAS enter biological organisms through ingestion, inhalation, and dermal exposures. The most common route of entry into the human body is the consumption of contaminated foods and drinking water. PFAS are found in products used daily, including kitchenware, food packaging and cosmetics, and they are readily detected in our food and water. Alarmingly, numerous studies have detected PFAS in public water systems at concentrations that significantly exceed advisory levels set by the USEPA. High levels of PFAS have also been detected in groundwater and drinking water in Europe, with the most extensive cases of residential PFAS exposures documented in Northern Italy following contamination of drinking water downstream of a fluorochemical plant.
Unlike many other POPs that are lipophilic (“fat-loving”) and thus tend to bioaccumulate in fatty tissues (adipose tissue and brain), PFAS, which possess chemical groups that provide both lipophilic and hydrophilic (“water-loving”) properties, preferentially associate with proteins in the blood and liver. The elimination rate of PFAS from the body varies between individual PFAS, but tends to be quite slow, with the biological half-life of some PFAS exceeding 10 years. This persistence in the human body is due in large part to the strength of their characteristic carbon-fluorine bond, which significantly limits PFAS metabolism. Rather, PFAS are slowly eliminated in unchanged primarily through urinary excretion. This process of elimination is further slowed by reabsorption of several PFAS compounds from the urine by renal transporters, which effectively increases the retention time of PFAS in the body.
Toxicological effects of PFAS
Identifying the toxicological effects of PFAS and characterizing the molecular mechanisms by which PFAS cause toxicity remain active areas of research. There are currently two major hypotheses as to how PFAS disrupt normal biological functions: (1) by modulating normal signaling through the peroxisome proliferator-activator receptor-ɑ (PPARɑ); and (2) via PPARɑ-independent mechanisms. PPARɑ is a nuclear receptor that regulates gene expression and functions as a central regulator of lipid metabolism. Both in vitro and in vivo studies indicate that PFAS can induce expression of PPARɑ-dependent genes, supporting the hypothesis that PFAS alters this critical metabolic pathway. However, experimental animal studies showed that genetic deletion of PPARɑ did not prevent all toxic outcomes, suggesting that PFAS exert effects independent of PPARɑ signaling. Recent research has demonstrated that PFAS are capable of associating with and influencing the function of biological substrates other than PPARα, which likely explains the diverse molecular effects of PFAS, which include not only dysregulated lipid metabolism, but also oxidative stress and impaired mitochondrial function. These molecular effects manifest as adverse impacts on numerous pathological processes across multiple organ systems (Figure 2).
Cancer:
Considerable research has examined the carcinogenic potential of PFAS, in particular the legacy PFAS, PFOS and PFOA. While the International Agency for Research on Cancer (IARC) has deemed PFOA, the most studied PFAS, a possible human carcinogen (group 2B), data from experimental and epidemiological studies are inconsistent. There remains limited epidemiological evidence associating PFAS exposures and cancer in the general population; however, such a relationship has been identified in individuals exposed to PFAS in an occupational setting. Clinical data revealed a positive correlation between serum concentrations of PFOA and renal cell carcinoma (RCC) risk, suggesting that PFOA is a likely renal carcinogen. Early clinical data from workers at DuPont facilities indicated an increased risk of kidney and testicular cancer following exposure to PFOA, while a 2022 clinical study linked exposure to PFOS to an increased risk of liver cancer. Studies in laboratory animals similarly demonstrated increased incidence of testicular, kidney, pancreatic, and liver cancer in rodents exposed to dietary PFOS. The carcinogenicity of other PFAS, especially new and emerging short-chain PFAS, such as GenX, has yet to be investigated.
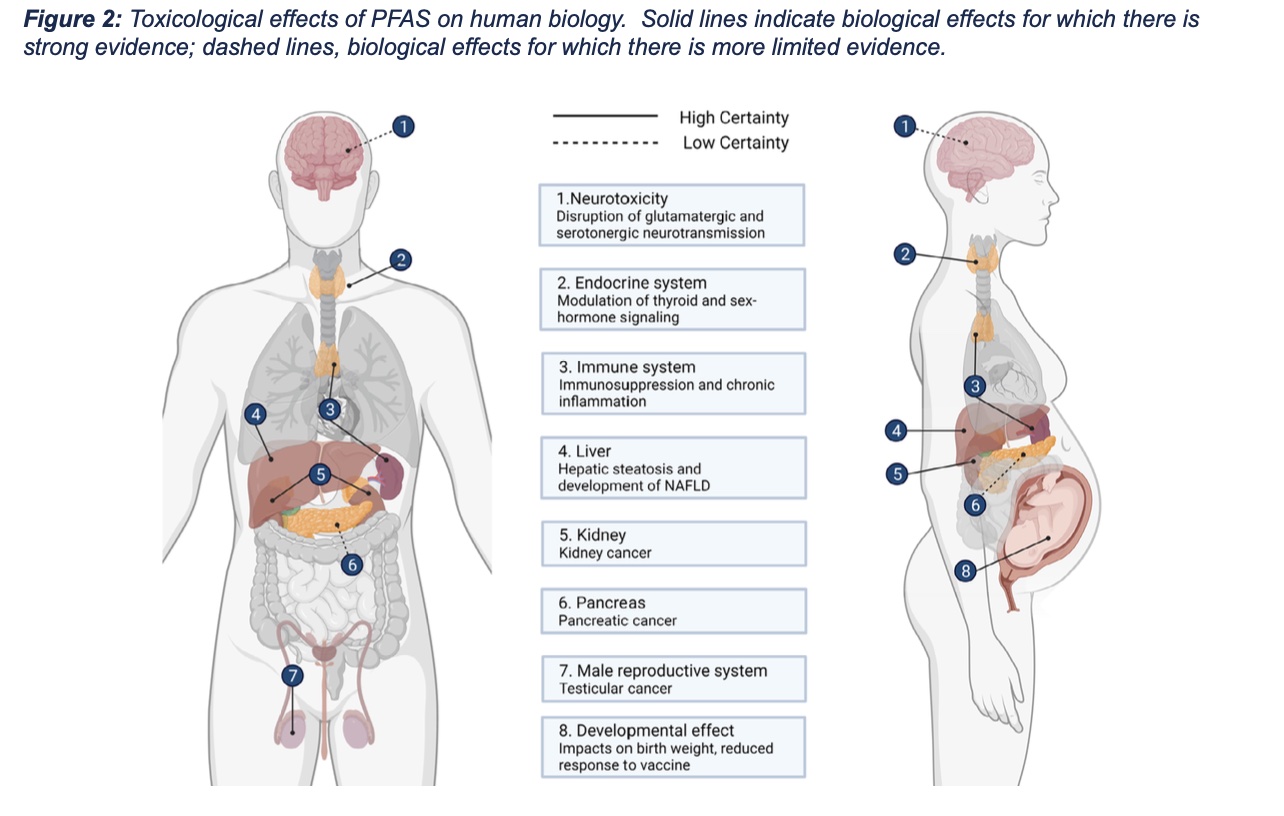
While the mechanisms underlying PFAS- associated carcinogenicity are poorly understood, it is generally recognized that PFAS are not DNA-reactive and, thus, not likely to be direct mutagens. Rather, emerging evidence points to epigenetic mechanisms, endocrine disruption and altered cellular metabolism as potential carcinogenic pathways.
PFAS Immunotoxicity:
Activation of inflammatory signaling pathways can broadly impact human physiology. Although the potential immunotoxic effects of PFAS are still under investigation, immunosuppression and chronic inflammation have been observed following PFAS exposure. A number of experimental animal studies have reported that PFAS turns on the canonical NLRP3 inflammasome signaling pathway, suggesting the potential for PFAS to induce inflammation. A 2016 National Toxicology Program (NTP) report concluded that both PFOA and PFOS are potential hazards to human immune health based on experimental and epidemiological data linking PFAS exposure to suppression of antibody responses. Further, developmental exposures to PFAS have been associated with increased risk for common infectious diseases in adolescents.
Neurotoxic effects of PFAS:
Experimental animal studies revealed elevated levels of PFOS in the brains of rat pups exposed in utero, while postmortem analysis detected PFAS in the brain and cerebral spinal fluid of individuals living in a PFAS-contaminated area. While PFAS have been readily detected in the brains of wildlife, laboratory animals and humans, the mechanisms by which PFAS enter the brain are poorly understood. Experimental and epidemiologic data suggest PFAS more readily access the brain in individuals with an impaired blood-brain barrier. Specifically, PFAS are believed to cross the blood-brain barrier when the tight junctions between endothelial cells that form brain capillaries are disrupted. Alternatively, there is evidence that PFAS may cross the blood-brain barrier via active transport, which normally functions to move molecules like glucose and amino acids from the blood into the brain. Accumulation of PFAS in the brain is associated with disruption of dopaminergic and glutamatergic neurotransmission, resulting in increased glutamate and catecholamine levels, and decreased dopamine levels. Some epidemiological data suggest PFAS exposure is linked to ADHD, perhaps due to disruptions in these neurotransmitter pathways. Additionally, limited experimental data demonstrate that developmental PFAS exposure exacerbated tau phosphorylation and amyloid β deposition in adult rat brains, suggesting the potential for PFAS to promote neurodegenerative pathologies, such as Alzheimer’s disease.
Adverse effects of PFAS on the liver:
Clinical, experimental and wildlife monitoring data indicate that PFAS accumulate in the liver. In laboratory animals, increased liver weight is a common outcome of PFAS exposure. Experimental animal and cell culture studies suggest that PFAS impair lipid trafficking and metabolism, resulting in a net increase in the synthesis and accumulation of lipids in the liver. Over time, these changes in lipid metabolism can progress to hepatic steatosis, also known as fatty liver disease, which adversely impacts liver function. The translational relevance of these experimental animal studies is suggested by meta-analyses of the epidemiological data that concluded there is an association between PFAS and increased risk of non-alcoholic fatty liver disease (NAFLD), a constellation of liver conditions not associated with alcohol consumption. These epidemiological data are consistent with data suggesting that PFAS promote clinical manifestations of NAFLD, including increased liver fat content, dysregulated glucose metabolism, and impaired lipid trafficking, evidenced by altered serum cholesterol levels.
Endocrine effects of PFAS:
Epidemiologic data have identified a positive correlation between serum PFAS concentrations and serum levels of thyroid hormone and thyroid stimulating hormone. Experimental data parallel these observations, with single high- dose or chronic low-dose exposures to PFAS shown to alter the expression of thyroid hormone-associated signaling molecules in rat models. It is suspected that these effects are PPARɑ-independent since in vitro studies indicated that PFAS induce expression of thyroid hormone-associated genes via direct interaction with the thyroid hormone receptor.
Given the promiscuous nature of PFAS interactions with biological systems, it is likely that PFAS interact with additional nuclear hormone receptors to alter signaling by diverse endocrine systems. In support of this possibility, limited in vitro data suggest an interaction between PFAS and androgen and estrogen receptors. Additionally, repeated high-dose PFAS exposures induced expression of estrogen receptor-associated genes in mice. Collectively, these data point to the potential for PFAS to influence sex- hormone signaling pathways in humans; however, more investigation is needed to confirm this effect.
Developmental toxicity of PFAS:
Several lines of evidence suggest the potential for developmental toxicity in humans exposed to PFAS. Clinical assessments indicate that PFAS readily cross the placenta, and that concentrations of PFAS are higher in the fetus than in maternal serum. Recent epidemiological studies found an association between maternal PFAS exposures and reduced fetal weight. Additionally, experimental data revealed frank developmental toxicity. Gestational PFAS exposure reduced offspring survival and weight gain, and delayed developmental milestones, like eye opening. The mechanisms underlying PFAS developmental toxicity remain unknown. It is speculated that the surfactant properties of PFAS interfere with proper lung development, leading to mortality. Conversely, other lines of evidence suggest lung-independent mechanisms of toxicity. There are also limited experimental data suggesting that exposure to PFAS during early development adversely impacts neurologic and immunologic outcomes in the exposed offspring.
Current regulatory environment
In the early 2000s, the USEPA first began regulating industrial PFAS production with the passage of two significant new use rules (SNURs) that required manufacturers, such as 3M and DuPont, to notify the USEPA when using or importing 75 PFAS that are in the process of being phased out. These SNURs represented critical action by the USEPA to address public health concerns surrounding PFAS, which triggered subsequent regulations by federal and state-level lawmakers to reduce PFAS contamination in the environment. In 2021, the USEPA outlined a three-year roadmap focused on research, regulation, and remediation of PFAS contamination. In 2022, the USEPA issued a new set of health advisories for PFAS in drinking water. Updated lifetime health advisories for PFOA and PFOS were lowered from a combined concentration of 70 parts per trillion (ppt) to 0.004 ppt for PFOA and 0.02 ppt for PFOS, indicating near-zero safe levels of PFAS exposure. Additionally, health advisories for GenX and FTBS, new generation replacements for PFOA and PFOS, were established for the first time at 10 and 2,000 ppt, respectively. Given the degree of PFAS contamination in drinking water sources, such health advisories are imperative for protecting public health. Various federal and state administrations have built upon the USEPA’s roadmap by investing $2 billion towards remediating PFAS contamination in drinking water across the U.S.
In addition to addressing water safety, the USEPA has continued to address PFAS usage in manufacturing, proposing a policy that would halt the processing and use of a group of 300 “inactive” PFAS, or chemicals that have not been used in years. The Department of Defense has also made increasing efforts towards reducing contamination from PFAS- containing firefighting foams used in and around military sites. A 2020 National Defense Authorization Act includes provisions that call for the phase-out of PFAS in firefighting foams, blood tests for military personnel, and monitoring of PFAS contamination in the military and nearby water systems. Such policies are not limited to regulatory bodies, as manufacturers have voluntarily begun to act. In a landmark pledge, 3M, one of the primary manufacturers of PFAS in the U.S., stated that it will discontinue all use of PFAS by 2025, while popular outdoor retailer REI plans to phase out both long and short-chain PFAS from its products by 2026.
Ultimately, however, the number and diversity of PFAS compounds complicate attempts to regulate this chemical class. While public outcry and emerging regulation have prompted the voluntary phase-out of many legacy PFAS, this has created a void in the market void that has been filled by the synthesis of novel short- chain fluorinated structures that skirt regulation targeting the longer-chain legacy perfluorinated chemicals. Efforts to characterize the toxicity of these new short- chain PFAS structures cannot keep pace with their development and production. The limited data that are available suggests that many of the newer short-chain PFAS derivatives are comparable to or even more toxic than the legacy PFAS compounds. In the U.S., regulatory frameworks are not designed to handle the wave of novel PFAS structures. Numerous stakeholders are advocating for moving away from regulating specific PFAS compounds towards regulating PFAS as a class based upon shared structural and chemical properties. These calls for changing this regulatory position of “chemical innocence” have met with varying degrees of success.
Overall, European nations have taken a more aggressive approach to PFAS management. The Stockholm Convention on POPs restricted the use of a large number of PFAS compounds as early as 2001. A mapping project recently completed by The Forever Pollution Project, a multi-national journalist organization, uncovered PFAS in over 17,000 sites across Europe and the UK, with particularly high concentrations of PFAS detected in ground and surface water systems at around 600 of those sites. Accordingly, in the past five years, the European Chemicals Agency, a regulatory arm of the European Union, has proposed to limit PFAS as a chemical class under the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, banning the use and production of more than 10,000 PFAS structures.
The future of PFAS
While regulation has finally begun to catch up with nearly a century of PFAS manufacturing and use, PFAS are ubiquitous in the environment, and even if all PFAS production were halted today, widespread PFAS contamination will continue to threaten wildlife and human health globally for many years, possibly decades. Public health officials are faced with the challenge of developing a two- pronged strategy for mitigating this toxicological crisis: 1) restrict further production and use; and 2) remove PFAS in the environment. The latter is a formidable task, given the persistence and scale of PFAS contamination.
On a more positive note, the voluntary phase- out of legacy PFAS has resulted in diminished PFAS levels in environmental and human samples. Unfortunately, this success story is offset by manufacturers’ shift to producing short-chain PFAS derivatives. While regulatory bodies are trying to keep pace with this chemical substitution by manufacturers, it is likely that the PFAS chemical landscape will continue to evolve faster than regulatory policies. Meanwhile, the development of strategies to reduce environmental PFAS contamination has gained traction. Novel approaches tested in recent years include the use of sorbent filters and chemical or biological degradation to remove PFAS from water.
Several filtration methodologies have been shown to successfully decontaminate PFAS-laden water under laboratory conditions. While encouraging, these filtration approaches do not destroy PFAS, resulting in the accumulation of PFAS-contaminated filter materials, which present significant disposal problems. To address this limitation, researchers are investigating techniques, both abiotic and biotic, to degrade these infamously inert chemicals. A number of studies suggest the feasibility of using chemical treatments to catalytically degrade PFAS. Various research groups are also investigating bacterial processes that may be capable of transforming and degrading PFAS. While promising, such engineered options for PFAS degradation are in their infancy and limited by the diversity of this chemical class. Regardless, this research offers hope for decreasing PFAS contamination in the future.
Conclusion
PFAS contamination is a global toxicological crisis. Environmental surveys have identified PFAS in every environmental matrix that has been examined, as well as in food and water supplies of hundreds of millions of people worldwide. Numerous epidemiological studies have identified associations between environmental PFAS exposures and a host of adverse health outcomes. Mechanistic research has only begun to elucidate how PFAS disrupt biological systems, but both clinical and experimental data indicate that nearly every organ system is impacted by PFAS, and that the developing organism may be particularly sensitive to PFAS toxicity. The global community has taken steps to curtail PFAS manufacturing and use, but the environmental persistence of these compounds creates challenges for long-term management of potential exposures. Reducing this ubiquitous PFAS contamination to protect human and ecosystem health will require the coordinated efforts of both regulators and researchers aimed at limiting the introduction of novel PFAS species, combined with the development of strategies to remove PFAS from environmental matrices.
You can read and download this full eBook “PFAS: The “New” Forever Chemicals” here


