Professor Colin Suckling of the University of Strathclyde discusses the heterocyclic compound known as Porphyrin, and the research he has undertaken
I’ve been struck recently by the enormous increase in and impact of the chemistry of heterocyclic compounds belonging to the family of compounds known as porphyrins. One paper in particular caught my eye; it is a structural and computational study of some new optoelectronic materials containing porphyrins (Adeninha et. al., J. Materials Chem. C. 2016, DOI: 10.1039/c6tc03957j) and this led me to look again at what has happened in porphyrin chemistry since I first engaged in it.
Understanding porphyrins
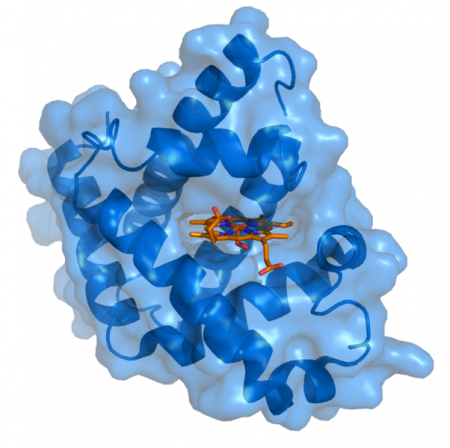
When I undertook my PhD research at the University of Liverpool in the 1970s the main interest in these heterocyclic compounds was their biological function as components of the oxygen-carrying proteins, haemoglobin and myoglobin, and the oxidation/reduction proteins known as cytochromes. In the following years the properties of these porphyrins and their host proteins were brought to a high level of understanding with the aid of both synthetic heterocyclic chemistry and X-ray crystallography (Figure 1).
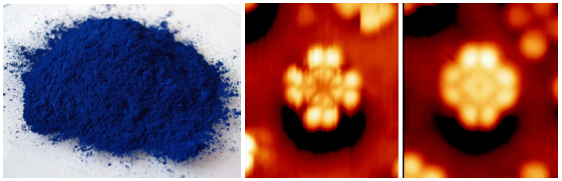
Many years before that the porphyrin relatives known as phthalocyanines had been used extensively as blue pigments in paints. Moreover different crystalline forms of phthalocyanines gave rise to different colours and again the value of X-ray crystallography shone through in the determination of structure (Figure 2).
Unlike the biologically active systems incorporating porphyrins, in which the porphyrins act singly and largely independently of another porphyrin component, the related phthalocycanines were found to form crystalline arrays in which many molecules of phthalocyanine formed uniformly oriented stacks or layers. What both of these classes of heterocyclic compounds have in common, however, is that they contain metal ions (respectively iron and copper), which make them able to take part in electron transfer reactions, and that they are coloured (respectively red and blue), which enables them to interact with visible light.
Together these properties mean that porphyrin metal complexes (as they are called) can both sense their environment by absorbing light and then do something with it through the metal ion. For these reasons and their very symmetrical geometric structure, porphyrins have become the key components of some of the most subtle exploitations of heterocyclic chemistry, in synthetic molecular machines, molecular electronics, in catalysis, and in tumour selective anti-cancer drugs. To emphasise the quality and significance of the science, the Nobel Prize in Chemistry for 2016 was awarded to Feringa, Stoddart, and Sauvage for their pioneering work on molecular machines. Not bad for one type of compound.
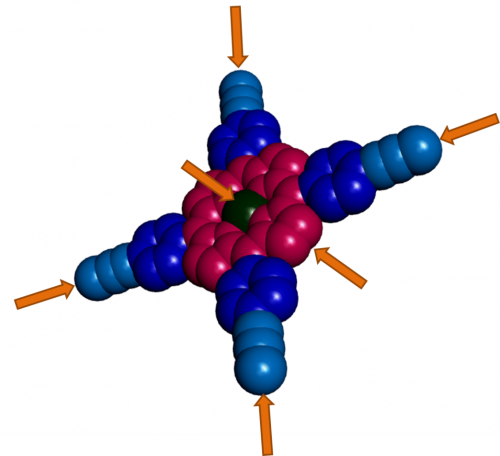
Figure 3 shows schematically how things can be put together. The porphyrin is shown in red with a metal ion in green at its centre. Attached to each edge in dark and light blue are components that can build a three dimensional molecular structure or connect with other parts of the molecular device by electron transfer. The orange arrows indicate the principal points of connection with other components of the device. Using appropriate chemical reactions and strategies it is possible to build molecular devices that range far beyond the natural functions for which porphyrins evolved.
Heterocyclic compound for smaller components
Molecular devices using porphyrins are part of a drive to find ways to minimise the size of components in electrical circuits which in turn will greatly reduce the demand for power. These are aspects of nanotechnology that can penetrate an enormous range of the artefacts that characterise the world of the early 21st century. It is cutting edge research that will take time to find its place in manufacturing industry.
We already know from inventions of the late 20th century, however, what a difference new molecular based technologies can make. I have in mind OLED technology which began in the late 1980s and is now a major technology in mobile phone and television screens. Indeed porphyrins and phthalocyanines have featured in OLED research. However the time required to develop research discoveries into innovations suggests that it may be another 20 years before we see the mass benefits of today’s cutting edge research. On the other hand, perhaps it will take less time to take advantage of the new chemistry and technology in fields like photodynamic therapy for cancer and proliferative disease where smaller quantities and long-term physical robustness are less of a challenge. Either way, the importance and potential impact of these research fields in applied heterocyclic chemistry is clear.
For further reading, the following might be helpful for those with a basic technical knowledge:
Perspective and technical articles on molecular electronics: https://www.nature.com/collections/gndyxpdxgw
Molecular electronics and nanoscale devices: https://pubs.acs.org/doi/abs/10.1021/acs.analchem.7b04533
Photodynamic therapy for cancer treatment:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6090489/
Porphyrins and catalysis: https://pubs.rsc.org/en/content/articlelanding/2018/cs/c7cs00582b#!divAbstract